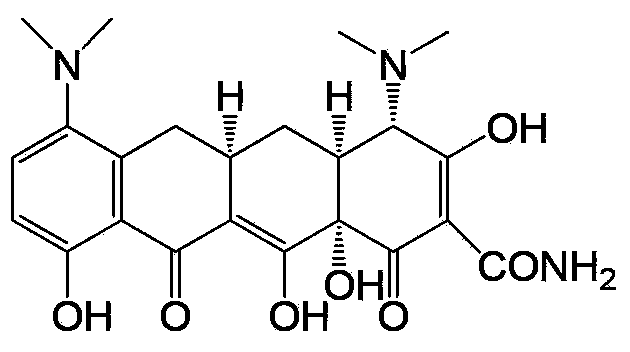
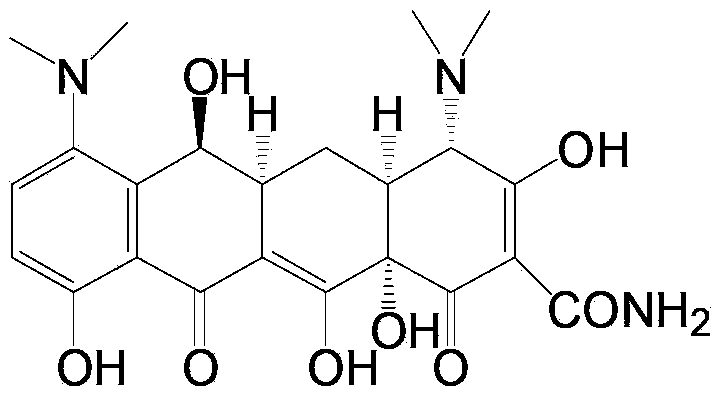
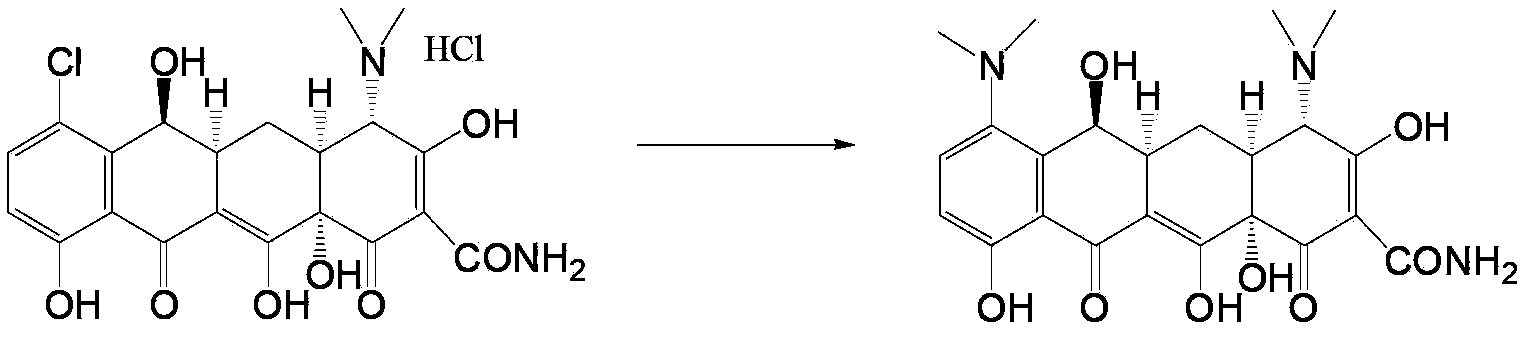
Preparation method and intermediate of minocycline
A technology of minocycline and tetracycline, which is applied in the preparation of organic compounds, carboxylic acid amide preparation, chemical instruments and methods, etc., and can solve the problems of high price and difficult availability of 7-aminocycline
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] In a 1000ml three-necked flask, add 50g of demethylaureomycin hydrochloride (content greater than 99%, mass percentage), add 500ml of DMF, add 10g of diisopropylimidazolium palladium chloride, and stir to dissolve at room temperature. Cool in an ice-water bath, add 30ml of 40% dimethylamine aqueous solution, and stir and react at 10°C to 15°C for 8 hours. HPLC detects the reaction solution, and the demethylactetracycline is 1.22%, and the reaction is terminated.
[0055] Add the above reaction solution into 6000ml of isopropanol, stir to precipitate a solid, then put it into a -20°C freezer, and freeze it for 8 to 10 hours. Filter, and wash the filter cake with 50 ml of isopropanol at -20°C. The filtrate is collected in a separate recovery bucket. The filter cake was dried under reduced pressure at 30°C-40°C for 8 hours to obtain 43.6 g of the product 7-dimethylamino-6-nortetracycline (M-M), yield: 91.1%, the product 7-dimethylamino- 6-nortetracycline (M-M) purity: 9...
Embodiment 2
[0060] In a 1000ml three-necked flask, add 50g of desmethyltetracycline hydrochloride (content greater than 99%), add 500ml of N-methylpyrrolidone, add 8g of tetrakis(triphenylphosphine)palladium, stir and dissolve at room temperature. Add 30ml of 40% dimethylamine aqueous solution, and stir the reaction at 45-55°C for 5 hours. HPLC detects the reaction solution, and the demethylactetracycline is 0.74%, and the reaction is terminated.
[0061]Add the above reaction solution into 6500ml of isopropanol, stir to precipitate a solid, then put it into a -20°C freezer, and freeze it for 8 to 10 hours. Filter and wash the filter cake with 50 ml of frozen isopropanol. The filtrate is collected in a separate recovery bucket. The filter cake was dried under reduced pressure at 30-40°C for 8 hours to obtain 44.3 g of the product 7-dimethylamino-6-nortetracycline (M-M), yield: 90.2%, the product 7-dimethylamino-6 - Nortetracycline (M-M) purity: 95.9%.
[0062] Add 44.3 g of 7-dimethyl...
Embodiment 3
[0066] In a 1000ml three-neck flask, add 50g of demethylaureomycin hydrochloride (content greater than 99%), add 500ml of DMPU, add 10g of bistriphenylphosphinepalladium dichloride, stir and dissolve at room temperature. Add 35ml of 40% dimethylamine aqueous solution, and stir and react at 20-25°C for 9 hours. HPLC detects reaction solution, and demethylactetracycline is 0.97%, finishes reaction.
[0067] Add the above reaction solution into 6000ml of isopropanol, stir to precipitate a solid, then put it into a -20°C freezer, and freeze it for 8 to 10 hours. Filter and wash the filter cake with 50 ml of frozen isopropanol. The filtrate is collected in a separate recovery bucket. The filter cake was dried under reduced pressure at 30-40°C for 8 hours to obtain 45.2 g of the product 7-dimethylamino-6-nortetracycline (M-M), yield: 92.3%, the product 7-dimethylamino-6 - Nortetracycline (M-M) purity: 96.8%.
[0068] Add 45.2 g of 7-dimethylamino-6-nortetracycline (M-M) into 550...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com