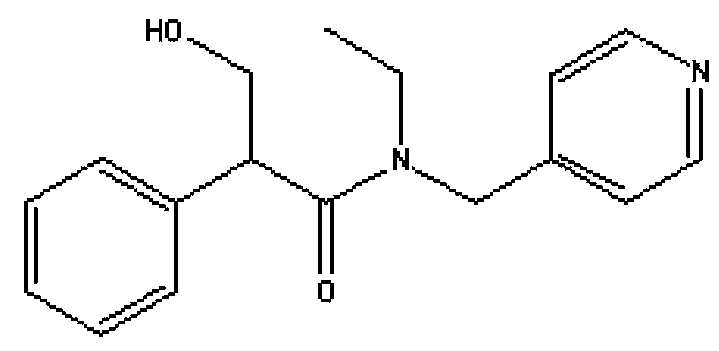
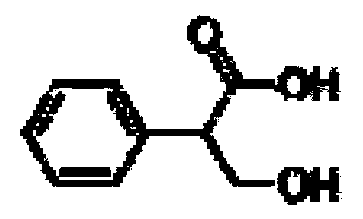
Method for separating tropicamide raceme
A racemate, tropicamide technology, applied in the direction of organic racemization, organic chemical methods, chemical instruments and methods, etc., to achieve the effect of precise medication
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Add 10g tropicamide racemate (35mmol) to 35ml (R)-(+)-1-phenylethanol, stir to dissolve, add dropwise 10ml from 1g(S)-(+)-ammonium lactate and 1.6 g(18.3mmol) (S)-(+)-lactic acid prepared into a methanol solution, after dripping, the temperature was raised to 65°C, and the methanol was distilled off. Let stand, lower the temperature to 18°C for crystallization, and filter.
[0058] The filter cake was dissolved in 20ml of water, 15ml of chloroform was added, the pH was adjusted to 9-10 with 3N sodium hydroxide solution while stirring, the layers were separated, the organic phase was washed twice with 10ml of water, and the chloroform was recovered by distillation. Dry under reduced pressure at 60°C for 2h to obtain 4.1g of pure (S)-(-)-topecaramide, [α]=-54.3°.
[0059] Add 30ml of water and 30ml of chloroform to the filtrate, adjust the pH to 1-2 with 2N sulfuric acid, separate the layers, and then extract and wash the aqueous phase twice with 10ml of chloroform. Add 25...
Embodiment 2
[0061] Add 10g tropicamide racemate (35mmol) to 30ml (R)-(+)-1-phenylethanol, stir to dissolve, add dropwise 10ml from 1g(S)-(+)-ammonium lactate and 1.6 g(18.3mmol) (S)-(+)-lactic acid prepared into a methanol solution, after dripping, the temperature was raised to 65°C, and the methanol was distilled off. Let stand, lower the temperature to 20°C for crystallization, and filter.
[0062] The filter cake was dissolved in 20ml water, 15ml chloroform was added, pH was adjusted to 9-10 with 3N sodium hydroxide solution while stirring, the layers were separated, the organic phase was washed twice with 10ml water, the chloroform was recovered by distillation, and dried under reduced pressure at 60℃ In 2h, 4.5 g of pure (S)-(-)-topecaramide was obtained, [α]=-49.7°.
[0063] Add 30ml of water and 25ml of chloroform to the filtrate, adjust the pH to 1-2 with 2N sulfuric acid, separate the layers, and then extract and wash the aqueous phase twice with 10ml of chloroform. Add 25ml of chlo...
Embodiment 3
[0065] Add 10g tropecaramide racemate (35mmol) to 40ml (R)-(+)-1-phenylethanol, stir to dissolve, add dropwise 10ml of 1g(S)-(+)-ammonium lactate and 1.6 g(18.3mmol) (S)-(+)-lactic acid prepared into a methanol solution, after dripping, the temperature was raised to 65°C, and the methanol was distilled off. Let stand, cool to 17°C for crystallization, and filter.
[0066] The filter cake was dissolved in 20ml water, 15ml chloroform was added, pH was adjusted to 9-10 with 3N sodium hydroxide solution while stirring, the layers were separated, the organic phase was washed twice with 10ml water, the chloroform was recovered by distillation, and dried under reduced pressure at 60℃ In 2h, 3.9g of pure (S)-(-)-topecaramide was obtained, [α]=-56.3°.
[0067] Add 30ml of water and 30ml of chloroform to the filtrate, adjust the pH to 1-2 with 2N sulfuric acid, separate the layers, and wash the aqueous phase twice with 10ml of chloroform respectively. Add 25ml of chloroform to the water ph...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com