Method for preparing electrolytes of all-vanadium flow battery
A technology of all-vanadium redox flow battery and electrolyte, which is applied in the field of preparation of liquid flow batteries, can solve problems such as unfavorable evaporation and crystallization, difficult removal of oxalic acid, and unstable sulfurous acid, so as to reduce energy consumption, benefit large-scale production, The effect of reducing production time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
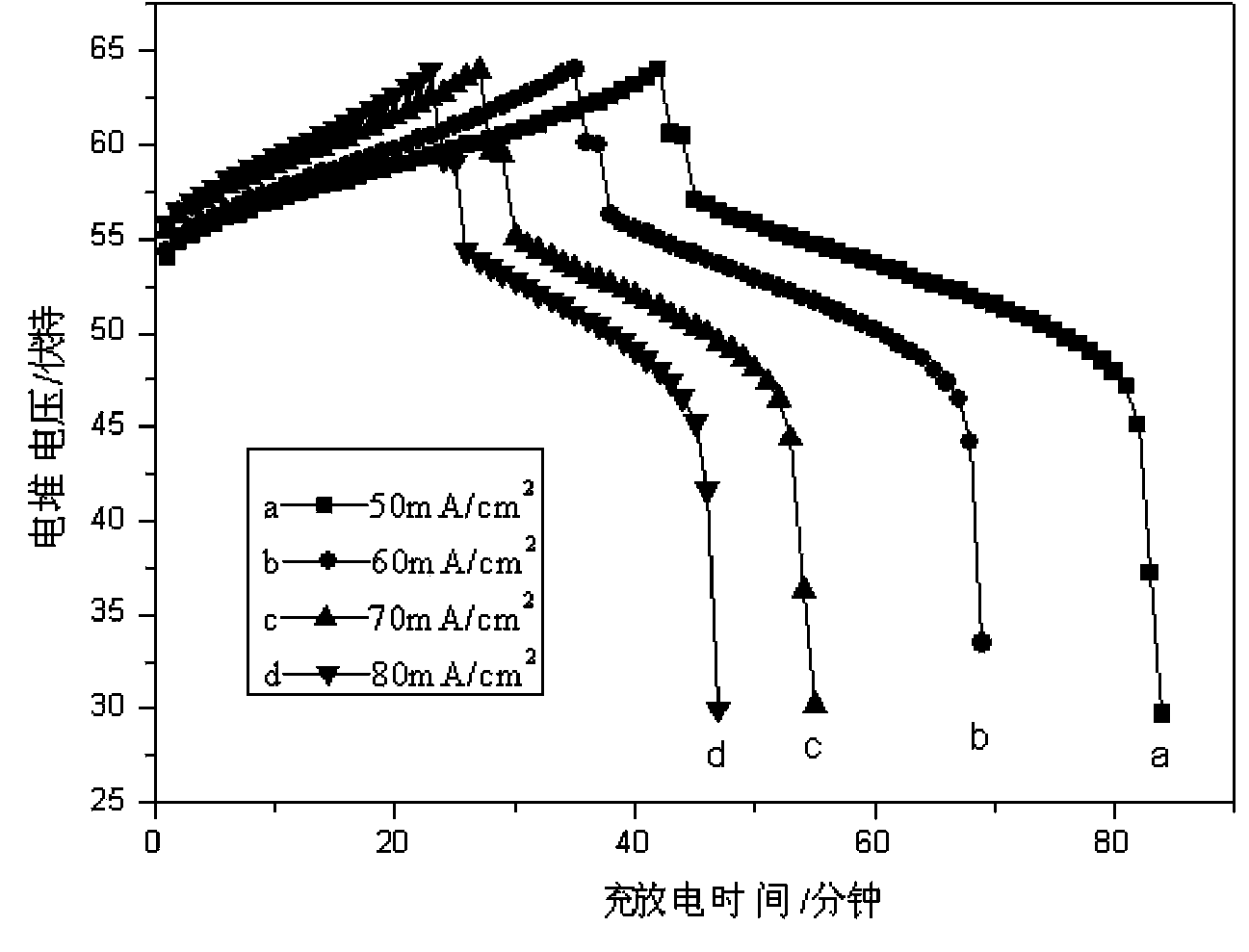
[0019] Take purity 99% H 2 2g, 99.8% pure V 2 o 5 18.2g, sintered at high temperature (80°C), the resulting solid (V 2 o 4 Powder, V 2 o 3 Powder) was dissolved with 9.8 g of concentrated sulfuric acid of 18.4 mol / liter to obtain an electrolyte solution that can be used for all-vanadium redox flow batteries. Put the prepared electrolyte into the electrolytic cell for charging and discharging experiments: the charging platform is 1.7 V, the discharging platform is 1.4 V, and in the assembled stack, the current density is 50 mA / cm 2 , the obtained current efficiency is 93.8%, the voltage efficiency is 88.3%, and the energy efficiency is 82.%.
Embodiment 2
[0021] Take purity 99% H 2 4g, 99.8% pure V 2 o 5 18.2g, sintered at high temperature (210°C), the resulting solid (V 2 o 4 Powder, V 2 o 3powder) was dissolved with 9.8 g of concentrated sulfuric acid of 10.0 mol / liter to obtain an electrolyte solution that can be used in an all-vanadium redox flow battery. Put the prepared electrolyte into the electrolytic cell for charging and discharging experiments: the charging platform is 1.7V, the discharging platform is 1.4V, and in the assembled stack, the current density is 60mA / cm 2 , the obtained current efficiency is 93.9%, the voltage efficiency is 86.1%, and the energy efficiency is 80.8%.
Embodiment 3
[0023] Take purity 99% H 2 3g, 99.8% pure V 2 o 5 18.2g, sintered at high temperature (500°C), the resulting solid (V 2 o 4 Powder, V 2 o 3 Powder) was dissolved in 9.8 g of concentrated sulfuric acid at 6 mol / L to obtain a vanadium battery electrolyte with a 1:1 ratio of trivalent vanadium and tetravalent vanadium. Put the prepared electrolyte into the electrolytic cell for charging and discharging experiments: the charging platform is 1.7V, the discharging platform is 1.4V, and in the assembled stack, the current density is 70mA / cm 2 , the obtained current efficiency is 94.9%, the voltage efficiency is 83.3%, and the energy efficiency is 79.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com