Non-aqueous electrolyte and lithium titanate battery
A non-aqueous electrolyte, lithium titanate battery technology, applied in the field of materials, can solve the problems of lithium dendrite piercing the separator, battery burning, safety hazards of lithium ion batteries, etc., and achieves good cyclability, suppression of gas swelling, and good rate. The effect of charge and discharge performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
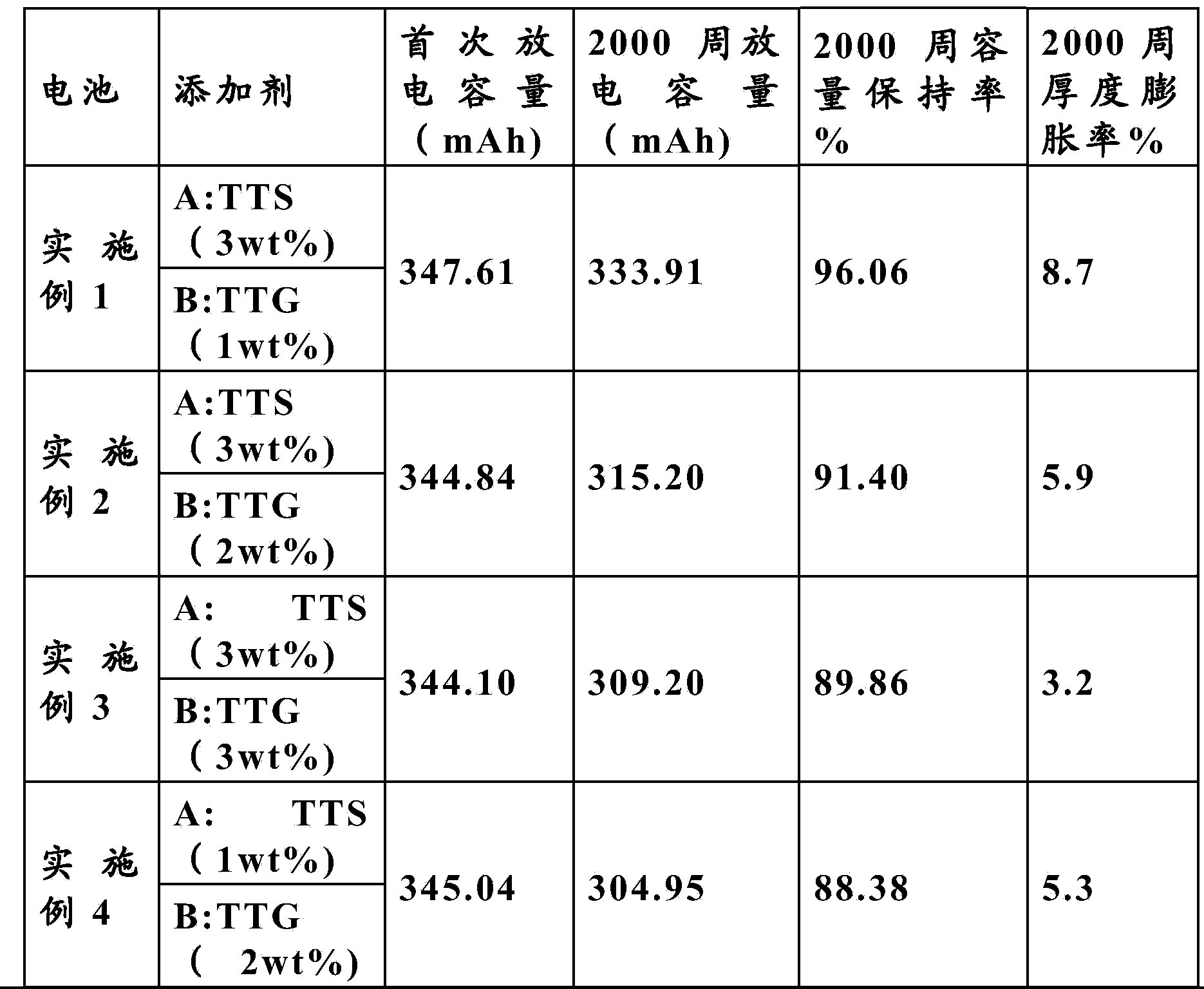
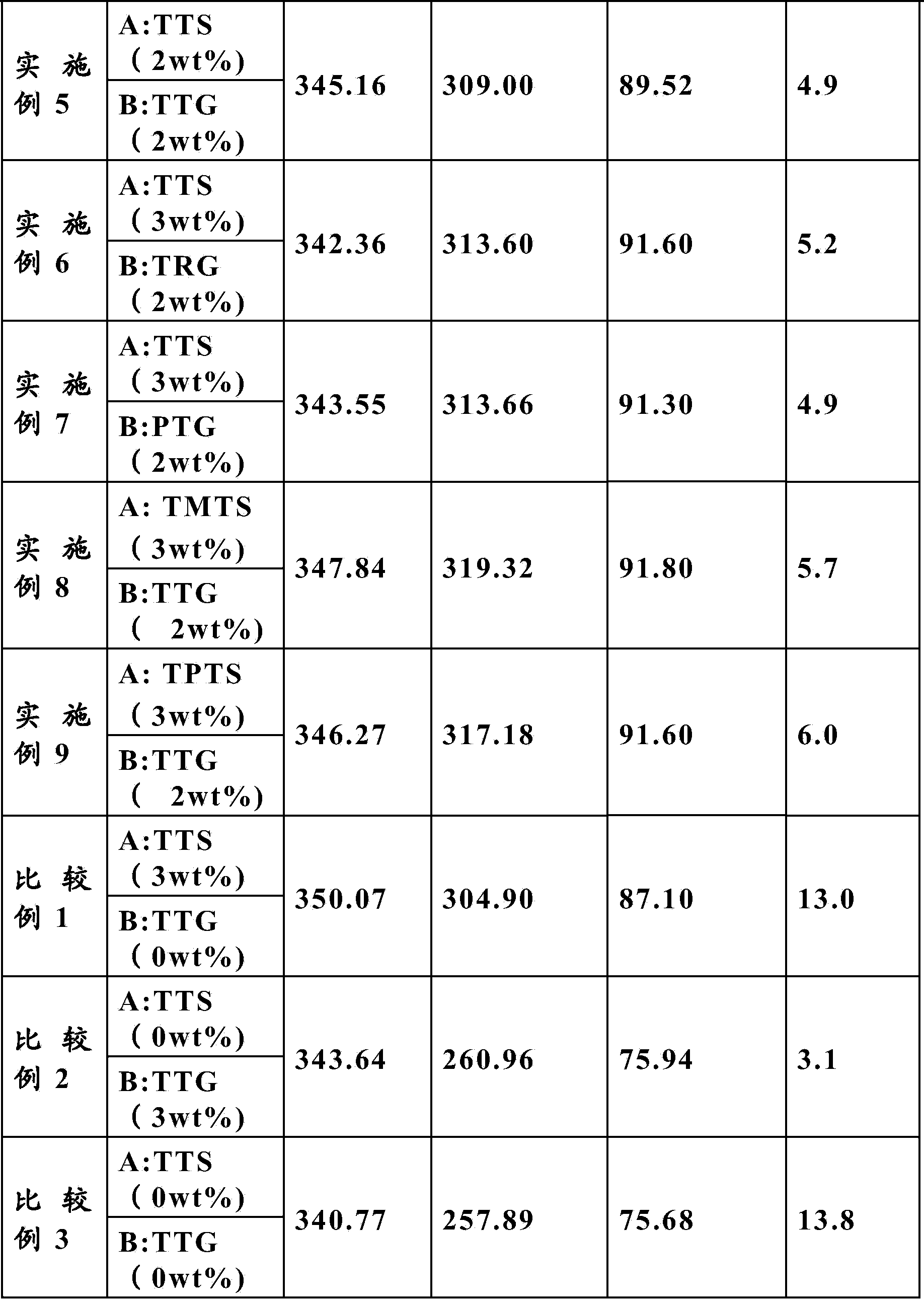
[0025] In a glove box filled with argon (H 2 O 6 ) In which, 3wt% of 3-thiocyanatopropyl triethoxysilane TETS (Triethoxy(3-thiocyanatopropyl)silane) and 1wt% of tetraethylene glycol dimethyl ether TTG are added to the electrolyte. (Tetraglyme).
[0026] The electrolyte was injected into a 340mAh NMC / LTO aluminum shell battery, and the battery was formed by charging and discharging at 0.1C, and then charging and discharging at 1C for 2000 weeks to test its capacity retention and thickness. The results are shown in Table 1.
Embodiment 2
[0028] In a glove box filled with argon (H 2 O 6 ) In which, 3wt% of 3-thiocyanatopropyltriethoxysilane TETS (Triethoxy(3-thiocyanatopropyl)silane) and 2wt% of tetraethylene glycol dimethyl ether TTG are added to the electrolyte (Tetraglyme).
[0029] The electrolytic solution described above was formed under the same conditions as in Example 1, and then a 1C charge-discharge cycle was performed under the same conditions as in Example 1. The results are shown in Table 1.
Embodiment 3
[0031] In a glove box filled with argon (H 2 O 6 ) In which, 3wt% of 3-thiocyanatopropyl triethoxysilane TETS (Triethoxy(3-thiocyanatopropyl)silane) and 3wt% of tetraethylene glycol dimethyl ether TTG are added to the electrolyte. (Tetraglyme).
[0032] The electrolytic solution described above was formed under the same conditions as in Example 1, and then a 1C charge-discharge cycle was performed under the same conditions as in Example 1. The results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com