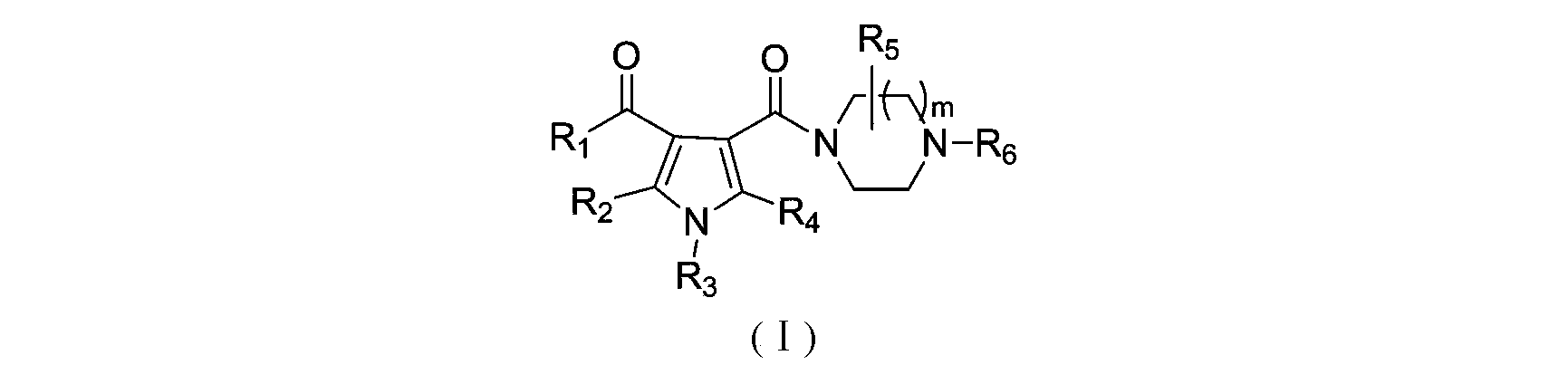
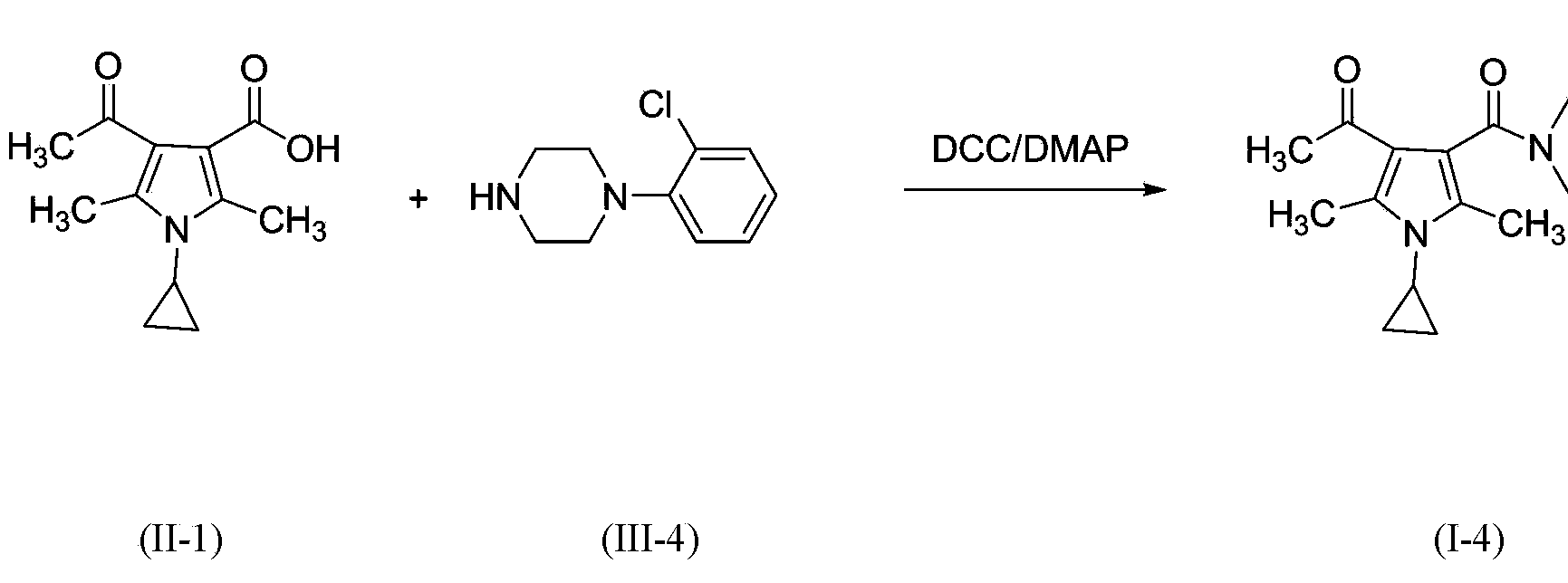
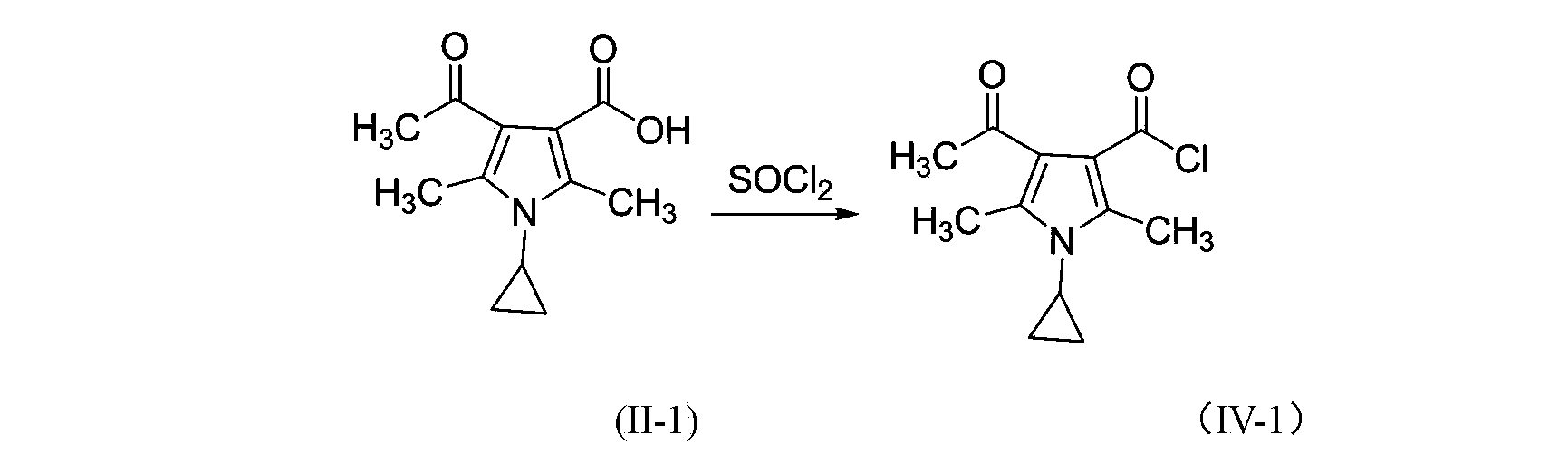
3-pyrrole carboxylic acid derivatives, and preparing method and application thereof
A compound and unsaturated technology, applied to 3-pyrrolecarboxylic acid derivatives, medicine, its preparation method and in the field of medicine, can solve the problems such as insufficient activity and side effect physicochemical properties of benzoazepine compounds, and achieve obvious antagonistic effect. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060]
[0061] II-1 (10g, 45 mmol) was placed in a 250mL reaction flask, and CH 2 Cl 2 (80mL) was stirred to dissolve, and intermediate III-1 (5.0g, 50mmol) was added in batches, followed by DMAP (6.2g, 50mmol) and HOBt (15g). After 0.5 h, DCC (15.8 g, 76.7 mmol) was added in batches to react for 16 h, and TLC detection showed that the reaction was complete (developer ethyl acetate).
[0062] The solvent was evaporated to dryness, ethyl acetate (50ml) was added, and the by-product DCU was removed by filtration. The filtrate was washed with saturated sodium carbonate and saline solution (50ml each), and the organic layers were combined, dried over anhydrous sodium sulfate, and left overnight. After filtration, the solvent was evaporated to dryness under reduced pressure to obtain a light yellow oil. The resulting product was purified by silica gel column chromatography to obtain 10.4 g of a white solid. The purity is 98.9% (HPLC normalization method), and the yield is 76...
Embodiment 2
[0064]
[0065] Put II-1 (10g, 45 mmol) in a 250mL reaction flask, add tetrahydrofuran (80mL) and stir to dissolve, add intermediate III-2 (6.5g, 50mmol) in batches, and then add DMAP (6.2g, 50mmol) and HOBt (15g), the reaction system turned light yellow after the addition, stirred at 60°C for 0.5h, added DCC (15.8g, 76.7mmol) in batches to continue the reaction for 12h, TLC detection showed that the reaction was complete (developer ethyl acetate).
[0066] The solvent was evaporated to dryness, ethyl acetate (50ml) was added, and the by-product DCU was removed by filtration. The filtrate was washed with saturated sodium carbonate and saline solution (50ml each), and the organic layers were combined, dried over anhydrous sodium sulfate, and left overnight. After filtration, the solvent was evaporated to dryness under reduced pressure to obtain a light yellow oil. The resulting product was purified by silica gel column chromatography to obtain 8.3 g of a white solid. The pu...
Embodiment 3
[0068]
[0069]
[0070] Put II-1 (10g, 45 mmol) in a 250mL reaction flask, add DMF (100mL) and stir to dissolve, add intermediate III-3 (8.1g, 50mmol) in batches, and then add DMAP (6.2g, 50mmol) and HOBt (15g), the reaction system turned light yellow after the addition, stirred at 80°C for 0.5h, added DCC (15.8g, 76.7mmol) in batches to continue the reaction for 6h, TLC detection showed that the reaction was complete (developer ethyl acetate).
[0071] The reaction solution was poured into 300ml of cold water, stirred, and a yellow solid was precipitated. Filter, dissolve the filter cake in ethyl acetate (50ml), filter to remove the insoluble by-product DCU, wash the filtrate with saturated sodium carbonate and saline solution (50ml each), combine the organic layers, dry over anhydrous sodium sulfate, and let stand overnight. After filtration, the solvent was evaporated to dryness under reduced pressure to obtain a light yellow oil. The obtained product was purified b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com