Application of matrine to preparation of medicament for treating CML
A technology for chronic myeloid and leukemia, applied in the fields of medicinal chemistry and cell biology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
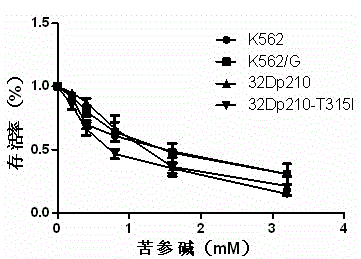
[0022] Example 1: Matrine inhibits the proliferation of CML cell lines and induces their apoptosis.
[0023] CML cell lines K562 (introduced from the Shanghai Cell Bank of the Chinese Academy of Sciences), K562 / G (gifted by Tianjin Blood Institute, China), 32Dp210, 32Dp210-T315I (gifted by Harbin Institute of Hematology) were selected as the research objects. Take K562, K562 / G, 32Dp210, 32Dp210-T315I in the logarithmic growth phase, wash once with RPMI 1640, add PMI 1640 medium containing 10% fetal bovine serum by volume fraction, inoculate in 96-well plate, adjust the cell concentration to 0.5- 1×10 5 / ml, the total volume of each well was 200μl, and were treated with 0, 0.2, 0.4, 0.8, 1.6, 3.2mM matrine, respectively. After 24 hours, add 20 μl of 5mg / ml MTT working solution to each well, mix well and incubate in a 37°C, 5% CO2 saturated humidity incubator for 4-6 hours. 2000 rpm, 5min, carefully absorb the supernatant with a pipette, add 200ml DMSO to each well, and bl...
Embodiment 2
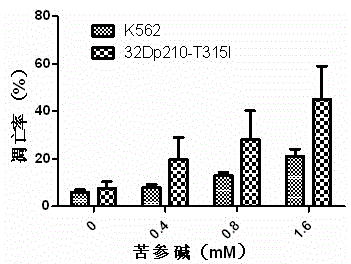
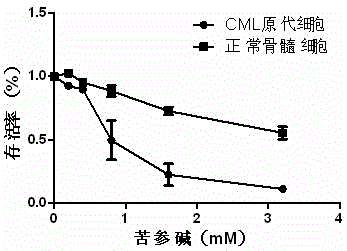
[0025] Example 2: Effects of matrine on growth inhibition and apoptosis induction of CML primary cells and normal bone marrow cells.
[0026] Collect 2-5ml of bone marrow or peripheral blood from patients with new-onset CML and normal people, centrifuge with lymphocyte separation medium density gradient at 2000rpm for 20min, absorb mononuclear cells, and wash 3 times with 1×PBS. Seed in 96-well plate, adjust the cell concentration to 5×10 5 / ml, the total volume of each well was 200μl, treated with 0, 0.2, 0.4, 0.8, 1.6, 3.2mM matrine respectively; inoculated in a 6-well plate, adjusted the cell concentration to 5×10 5 / ml, the final volume was 5ml, and they were treated with 0, 0.4, 0.8, 1.6mM matrine, respectively. After 24 hours, the growth inhibitory effect was detected by MTT method, and the cell apoptosis was detected by Annexin V / PI double staining flow cytometry. All experiments were repeated three times. see results image 3 and Figure 4 , showing that matrine h...
Embodiment 3
[0027] Example 3: Matrine enhances the sensitivity of K562 to imatinib.
[0028] Take K562 and 32Dp210-T315I in the logarithmic growth phase, seed them in 96-well plates, and adjust the cell concentration to 5×10 5 / ml, the total volume of each well is 200μl, and they are treated with 0.4uM imatinib, 0.8mM matrine alone and in combination. After 24 hours, growth inhibition was detected by MTT assay. All experiments were repeated three times. see results Figure 5 , showing that matrine significantly enhanced the sensitivity of K562 to imatinib, matrine single-drug group and combination group, imatinib single-drug group and combination group had statistical differences (p0.05).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com