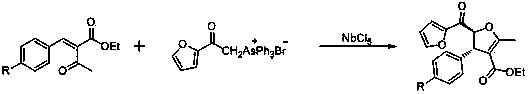Method for preparing 2-furan formyl-3-aryl-4-ethoxycarbonyl-5-methyl-anti-form-2 and 3-dihydrofuran under catalytic action of niobium chloride
A technology for brominating furoyl methyl triphenylarsine and furoyl group is applied in the field of chemical raw material synthesis to achieve the effects of easy separation and purification, mild reaction conditions and high stereoselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Prepare 2-furoyl-3-phenyl-4-ethoxycarbonyl-5-methyl-trans-2,3-dihydrofuran according to the following steps:
[0028] (a) Control the amount of furoylmethyltriphenylarsine bromide: 2-ethoxycarbonyl-3-phenyl-ethyl acrylate: niobium pentachloride: dichloromethane at a ratio of 1:1: 2:2;
[0029] (b) Put the ratio of the amount of each substance weighed in step (a) into the reaction container at the same time, stir until each component is fully dissolved, control the reaction temperature to 0 ~ 40 ℃, and the reaction time is 5 to 6 hours to obtain the reaction solution;
[0030] (c) Pour the reaction solution prepared in step (b) into cold water, crystallize and filter, and dry to obtain 2-furoyl-3-aryl-4-ethoxycarbonyl-5-methyl-resin Formula - 2,3-dihydrofuran.
[0031] The product yield of the obtained 2-furoyl-3-phenyl-4-ethoxycarbonyl-5-methyl-trans-2,3-dihydrofuran was 63%.
[0032] The obtained invention product is subjected to NMR detection, NMR data: 1 H NMR (...
Embodiment 2
[0034] This example prepares 2-furoyl-3-phenyl-4-ethoxycarbonyl-5-methyl-trans-2,3-dihydrofuran according to the following steps:
[0035] (a) Control the amount of furoylmethyltriphenylarsine bromide: 2-ethoxycarbonyl-3-phenyl-ethyl acrylate: niobium pentachloride: dichloromethane at a ratio of 1:2: 2:4;
[0036] (b) Put the ratio of the amount of each substance weighed in step (a) into the reaction container at the same time, stir until each component is fully dissolved, control the reaction temperature to 0-40 ℃, and the reaction time to 5-6 hours to get the reaction solution;
[0037] (c) Pour the reaction solution obtained in step (b) into cold water, crystallize and filter, and dry to obtain 2-furoyl-3-phenyl-4-ethoxycarbonyl-5-methyl-trans -2,3-dihydrofuran. The yield of the obtained 2-furoyl-3-phenyl-4-ethoxycarbonyl-5-methyl-trans-2,3-dihydrofuran was 73%.
[0038] The obtained invention product is subjected to NMR detection, NMR data: 1 H NMR (400 MHz, CDCl3 ) ...
Embodiment 3
[0040] Prepare 2-furoyl-3-phenyl-4-ethoxycarbonyl-5-methyl-trans-2,3-dihydrofuran according to the following steps:
[0041] (a) Control the amount of furoylmethyltriphenylarsine bromide: 2-ethoxycarbonyl-3-phenyl-ethyl acrylate: niobium pentachloride: dichloromethane at a ratio of 1:2: 2:4;
[0042] (b) Stir the furoylmethyltriphenylarsine bromide, 2-ethoxycarbonyl-3-phenyl-ethyl acrylate and dichloromethane to fully dissolve, then slowly add pentachloro Niobium chloride was reacted, the reaction temperature was controlled to be 0-40°C, and the reaction time was 3-4 hours to obtain a reaction solution;
[0043] (c), pour the reaction solution obtained in step (b) into cold water, crystallize and filter, and dry to obtain 2-furoyl-3-phenyl-4-ethoxycarbonyl-5-methyl- Formula - 2,3-dihydrofuran. The product yield of the obtained 2-furoyl-3-phenyl-4-ethoxycarbonyl-5-methyl-trans-2,3-dihydrofuran was 71%.
[0044] NMR data: 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 1.04 (t, J...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com