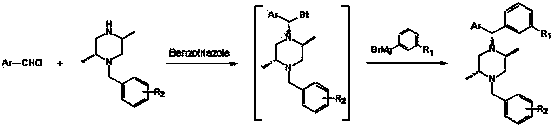
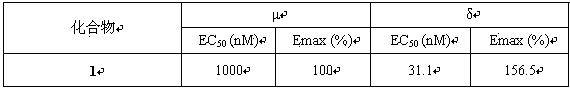Diaryl methyl piperazine compounds containing saturated nitrogen heterocyclic amide and application thereof
A compound, dimethyl technology, applied in the field of diarylmethylpiperazine compounds, can solve the problems of poor metabolic stability, limited clinical application value, and inability to take oral administration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
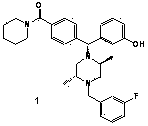
[0087] Example 1: 4-((α-R)-α-((2S,5R)-4-(3-fluorobenzyl)-2,5-dimethyl-1-piperazinyl)-3-hydroxybenzyl)benzene Preparation of formyl-1-piperidine
[0088]
[0089] The specific synthesis method is as follows:
[0090] Step 1, the preparation of (4-formyl)benzoyl-1-piperidine: take 4-formylbenzoic acid (50.0 g, 333.3 mmol), dissolve in dichloromethane (500 ml), and cool to At about 0°C, slowly add thionyl chloride (78.6 g, 666.6 mmol) dropwise, stir, remove the ice bath after the dropwise addition, and heat to reflux until the reaction is complete; cool down to room temperature, and concentrate the reaction solution to obtain 4-formylbenzene Formyl chloride, and then under ice-bath conditions, piperidine (28.26 g, 333.3 mmol) dissolved in dichloromethane (250 ml) was transferred to 4-formylbenzoyl chloride, stirred for 1 h, added water (500 ml), separate the organic phase, dry the organic phase with anhydrous sodium sulfate, and concentrate to obtain 50.0 g of oily matter,...
Embodiment 2
[0100] Example 2: 4-((α-R)-α-((2S,5R)-4-benzyl-2,5-dimethyl-1-piperazinyl)-3-hydroxybenzyl)benzoyl-1- Preparation of piperidine
[0101]
[0102] Synthetic method is the same as embodiment 1, and difference is that step two, five:
[0103] Step 2. Preparation of (2R,5S)-1-benzyl-2,5-dimethylpiperazine: 1-benzyl-2, 5-Dimethylpiperazine racemate, yield 93%; and then obtained (2R,5S)-1-benzyl-2,5-dimethylpiperazine through chiral tartaric acid salt crystallization resolution, yield 37% %;
[0104] 1 H NMR (400 MHz, CDCl 3 ) δ 7.22-7.32 (m, 5H), 4.10 (d, J = 13.5 Hz, 1H), 3.09 (d, J = 13.5 Hz, 1H), 2.91 (dd, J = 12.1, 3.1 Hz, 1H), 2.83-2.74 (m, 1H), 2.70-2.60 (m, 2H), 2.28-2.17 (m, 1H), 1.63 (dd, J = 11.0, 10.3 Hz, 1H), 1.49 (br, 1H), 1.14 (d, J = 6.0 Hz, 3H), 0.94 (d, J = 6.2 Hz, 3H);
[0105] Step 5, 4-((α-R)-α-((2S,5R)-4-benzyl-2,5-dimethyl-1-piperazinyl)-3-hydroxybenzyl)benzoyl - Preparation of 1-piperidine: Using (2R,5S)-1-benzyl-2,5-dimethylpiperazine as...
Embodiment 3
[0107] Example 3: 4-((α-R)-α-((2S,5R)-4-(3-chlorobenzyl)-2,5-dimethyl-1-piperazinyl)-3-hydroxybenzyl)benzene Preparation of formyl-1-piperidine
[0108]
[0109] Synthetic method is the same as embodiment 1, and difference is that step two, five:
[0110] Step 2. Preparation of (2R,5S)-1-(3-chlorobenzyl)-2,5-dimethylpiperazine: using 3-chlorobenzyl chloride as raw material, using the method described in step 2 of Example 1 1-(3-Chlorobenzyl)-2,5-dimethylpiperazine racemate was obtained with a yield of 92%; then, (2R,5S)-1-(3- Chlorobenzyl)-2,5-dimethylpiperazine, yield 35%;
[0111] 1 H NMR (400 MHz, CDCl 3 ) δ 7.26-7.22 (m,2H), 7.06-7.04 (m, 2H), 4.07 (d, J = 13.7 Hz, 1H), 3.06 (d, J = 13.7 Hz, 1H), 2.91 (dd, J = 12.1, 3.0 Hz, 1H), 2.80-2.78 (m, 1H), 2.65-2.59 (m, 2H), 2.24-2.23 (m, 1H), 1.76 (br, 1H), 1.65 (t, J = 10.7 Hz, 1H), 1.10 (d, J = 6.1 Hz, 3H), 0.94 (d, J = 6.4 Hz, 3H).
[0112] Step five, 4-((α-R)-α-((2S,5R)-4-(3-chlorobenzyl)-2,5-dimethyl-1-p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com