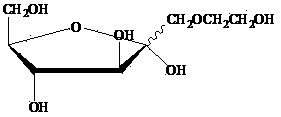
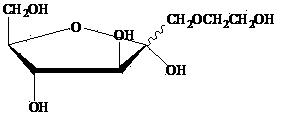
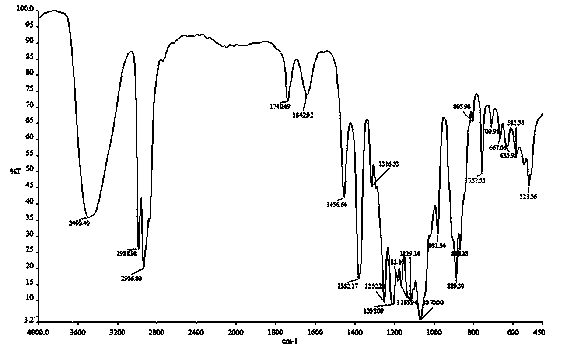
Tobacco humectant 1-O-hydroxyethyl-D-fructose and preparation method thereof
The technology of a moisturizing agent for cigarettes and hydroxyethyl is applied in the field of moisturizing agent 1-O-hydroxyethyl-D-fructose for cigarettes and its preparation, and can solve the problem of no moisture-proof effect, weak binding ability of the moisturizing agent, Problems such as low temperature and easy solidification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The preparation method of 1-O-carboxymethyl-D-fructofuranose, it comprises the steps:
[0031] (1) Preparation of 1-O-hydroxyethyl-2,3:4,5-di-O-isopropylidene-α-D-fructopyranose (Ⅱ)
[0032] Weigh 6.0 g of 1-O-carboxymethyl-2,3:4,5-di-O-isopropylidene-α-D-fructopyranose tert-butyl ester (I) into a 250 mL three-necked flask, add 48 mL The tetrahydrofuran (THF), magnetic stirring to dissolve, and then nitrogen protection, ice-water bath cooling to below 10 ℃ (preferably between 0-10 ℃), and then slowly add LiAlH 4 0.024mol, raise the temperature and react at 10°C for 2.5h, TLC monitors the entire reaction process (V 石油醚 : V 乙酸乙酯 =3: 2), and then slowly dropwise added 12mL of 70% ethyl acetate aqueous solution by volume to end the reaction, filtered the precipitate, and concentrated under reduced pressure to obtain 3.45g of colorless transparent viscous liquid (II), with a yield of 70.65%.
[0033] (2) Preparation of 1-O-hydroxyethyl-D-fructose (Ⅲ)
[0034] Add 1g of p...
Embodiment 2
[0036] The preparation method of 1-O-carboxymethyl-D-fructofuranose, it comprises the steps:
[0037] (1) Preparation of 1-O-hydroxyethyl-2,3:4,5-di-O-isopropylidene-α-D-fructopyranose (Ⅱ)
[0038] Weigh 6.0 g of 1-O-carboxymethyl-2,3:4,5-di-O-isopropylidene-α-D-fructopyranose tert-butyl ester (Ⅰ) into a 250 mL three-necked flask, add 60 mL The tetrahydrofuran (THF), magnetic stirring to dissolve, and then nitrogen protection, ice-water bath cooling to below 10 ℃ (preferably between 0-10 ℃), and then slowly add LiAlH 4 0.03mol, raise the temperature and react at 16°C for 3.5h, TLC monitors the entire reaction process (V 石油醚 : V 乙酸乙酯 =3: 2), then slowly dropwise added 19 mL of 50% by volume ethyl acetate aqueous solution to end the reaction, filtered the precipitate, and concentrated under reduced pressure to obtain 4.40 g of a colorless transparent viscous liquid (II), with a yield of 90.26%.
[0039] (2) Preparation of 1-O-hydroxyethyl-D-fructose (Ⅲ)
[0040] Add 1g of pr...
Embodiment 3
[0042] The preparation method of 1-O-carboxymethyl-D-fructofuranose, it comprises the steps:
[0043] (1) Preparation of 1-O-hydroxyethyl-2,3:4,5-di-O-isopropylidene-α-D-fructopyranose (Ⅱ)
[0044] Weigh 6.0 g of 1-O-carboxymethyl-2,3:4,5-di-O-isopropylidene-α-D-fructopyranose tert-butyl ester (Ⅰ) into a 250 mL three-necked flask, add 90 mL The tetrahydrofuran (THF), magnetic stirring to dissolve, and then nitrogen protection, ice-water bath cooling to below 10 ℃ (preferably between 0-10 ℃), and then slowly add LiAlH 4 0.033mol, raise the temperature and react at 25°C for 5h, TLC monitors the entire reaction process (V 石油醚 : V 乙酸乙酯 =3: 2), and then slowly dropwise added 24mL of 30% by volume ethyl acetate aqueous solution to end the reaction, filtered the precipitate, and concentrated under reduced pressure to obtain 4.38g of colorless transparent viscous liquid (II), with a yield of 89.72%.
[0045] (2) Preparation of 1-O-hydroxyethyl-D-fructose (Ⅲ)
[0046] Add 1g of produ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com