Preparation method of decoquinate dry suspension
A decoquinate and dry suspension technology, applied in the field of preparation of decoquinate dry suspension, can solve the problems of inability to administer by drinking water, complicated preparation process, inconvenient use, etc., and achieve convenient clinical drinking water. The effect of simple administration and preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
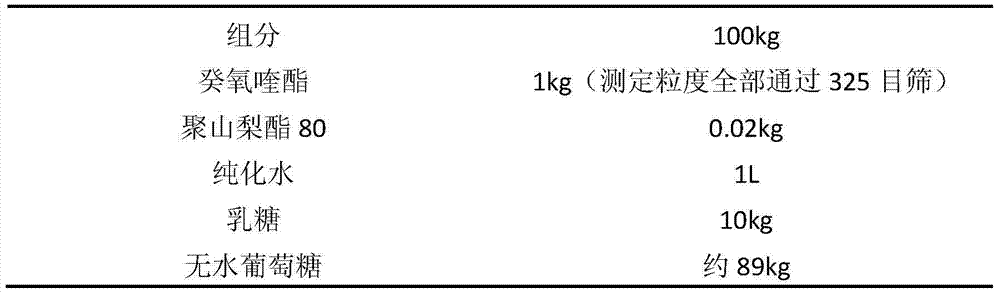
[0032] Embodiment 1: Decoquinate dry suspension formulation:
[0033]
[0034] Preparation method:
[0035] (1) Weigh 0.02kg of Tween 80, add it to 1L of water, stir evenly, add 1kg of decoquinate, stir to make it evenly dispersed, and it is Suspension A;
[0036] (2) Add the suspension A into the lactose and stir to make a soft material;
[0037] (3) Granulate the above-mentioned soft materials, dry them (directly dry them in a discharge heat blast drying oven, and control the temperature at about 60°C), crush them through a 60-mesh sieve, and obtain 10.92kg of powder with a measured content of 9.1%, adding Anhydrous glucose 88.5kg is mixed evenly, and packaged separately to obtain the product.
Embodiment 2
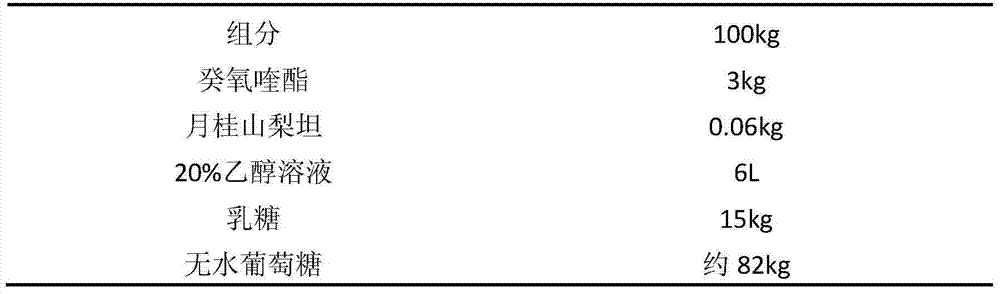
[0038] Embodiment 2: prescription of decoquinate dry suspension:
[0039]
[0040] Preparation method:
[0041] (1) Weigh 0.06g of sorbitan laurel, add it to 6L of 20% ethanol aqueous solution, stir well, add 3 kg of decoquinate, stir to make it evenly dispersed, and it is suspension A;
[0042] (2) Homogenize the suspension A twice through a high-pressure homogenizer at a pressure of 700-1000bar;
[0043] (3) Add the above solution into lactose and stir to make a soft material;
[0044] (4) Granulate the above-mentioned soft material, dry (boiling drying, temperature controlled at about 60°C), crush through a 60-mesh sieve to obtain 17.65kg of powder with a measured content of 17.0%, add 82.35kg of anhydrous glucose and mix well, Subpackage, that is.
Embodiment 3
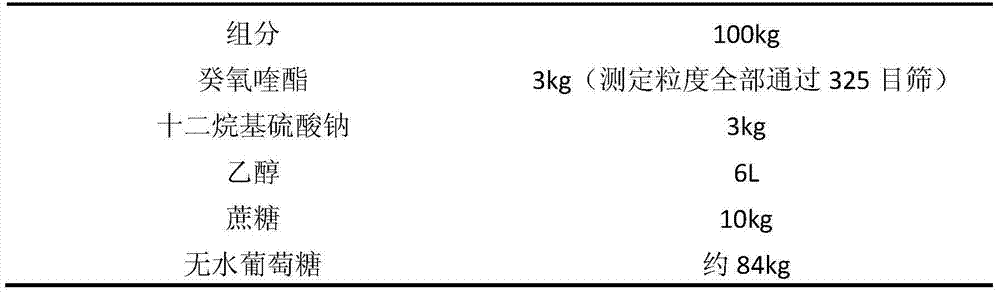
[0045] Embodiment 3: prescription of decoquinate dry suspension:
[0046]
[0047] Preparation method:
[0048] (1) Weigh 3kg of sodium lauryl sulfate, add it to 6L of ethanol, stir evenly, add 3kg of decoquinate, stir to make it evenly dispersed, and it is suspension A;
[0049] (2) Add the above solution into sucrose and stir to make a soft material;
[0050] (3) Granulate the above-mentioned soft material, dry (boiling drying, temperature controlled at about 60°C), crush through a 60-mesh sieve to obtain 15.59kg of powder with a measured content of 18.78%, add 84.41kg of anhydrous glucose and mix well, Subpackage, that is.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com