2-aryl benzothiazole compound with high affinity with a(BETA) plaque and preparation method and application thereof
A benzothiazole and compound technology, applied in the field of 2-arylbenzothiazole compounds, can solve the problem of lack of Aβ molecular probes, and achieve the effect of low initial brain uptake and fast blood clearance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1B
[0058] The synthesis of embodiment 1BTDPA and BTIDA compounds
[0059] 1. Synthetic intermediate 1
[0060] The compound 2-(4-(methylamino)phenyl)benzothiazole-6-hydroxyl (259.9mg, 1.0mmol) and K 2 CO 3 (429.9mg, 3.1mmol) was dissolved in 20mL of acetonitrile, after reflux reaction at 90°C for 2h, 1,3-dibromopropane (606.7mg, 2.7mmol) was added, and the reaction was continued for 3h, the solvent was rotary evaporated, and dichloromethane was added for suction filtration Potassium carbonate was removed, spin-dried, and petroleum ether was added to precipitate a pale yellow solid. The product was obtained by suction filtration. The structure was as follows, and the yield was 79.8%. 1 H NMR (400MHz, CDCl 3 )δ7.88(d,J=8.7Hz,2H),7.86(d,J=8.8Hz,1H),7.33(d,J=2.4Hz,1H),7.04(dd,J=8.9,2.5Hz, 1H), 6.65(d, J=8.7Hz, 2H), 4.17(t, J=5.8Hz, 2H), 3.64(t, J=6.4Hz, 2H), 2.91(s, 3H), 2.36(p, J=6.1Hz, 2H).
[0061]
[0062] 2. Synthetic intermediate 2
[0063] Intermediate 2 is prepared ...
Embodiment 2
[0145] Preparation of Example 2 Tc-99m-labeled BTDPA and BTIDA Compounds
[0146] The BTDPA and BTIDA compounds prepared in the above examples of the present invention can be labeled with the radionuclide Tc-99m by conventional methods in the prior art. For example, the compounds BT-3-IDA-Re, BT-5-DPA-Re and BT-6-IDA-Re will be described as examples.
[0147] The reaction route between Tc-99m-labeled BTDPA and BTIDA is as follows:
[0148]
[0149] 29. Labeled compound BT-5-DPA-Re
[0150] Put 5mg of sodium borohydride, 4mg of sodium carbonate and 20mg of sodium potassium tartrate into a 10mL penicillin vial, seal it, and vent the air with CO gas for 15min, add 3mL of sodium pertechnetate eluent, and place it in a water bath at 75°C for 30min , cooled to room temperature. Take 1mL [ 99m Tc(CO) 3 (H 2 O) 3 ] + Add 10mL of penicillin vial to adjust the pH value to about 7, then add 0.5mg of labeled intermediate 15, add appropriate amount of ethanol to dissolve it, pla...
Embodiment 32
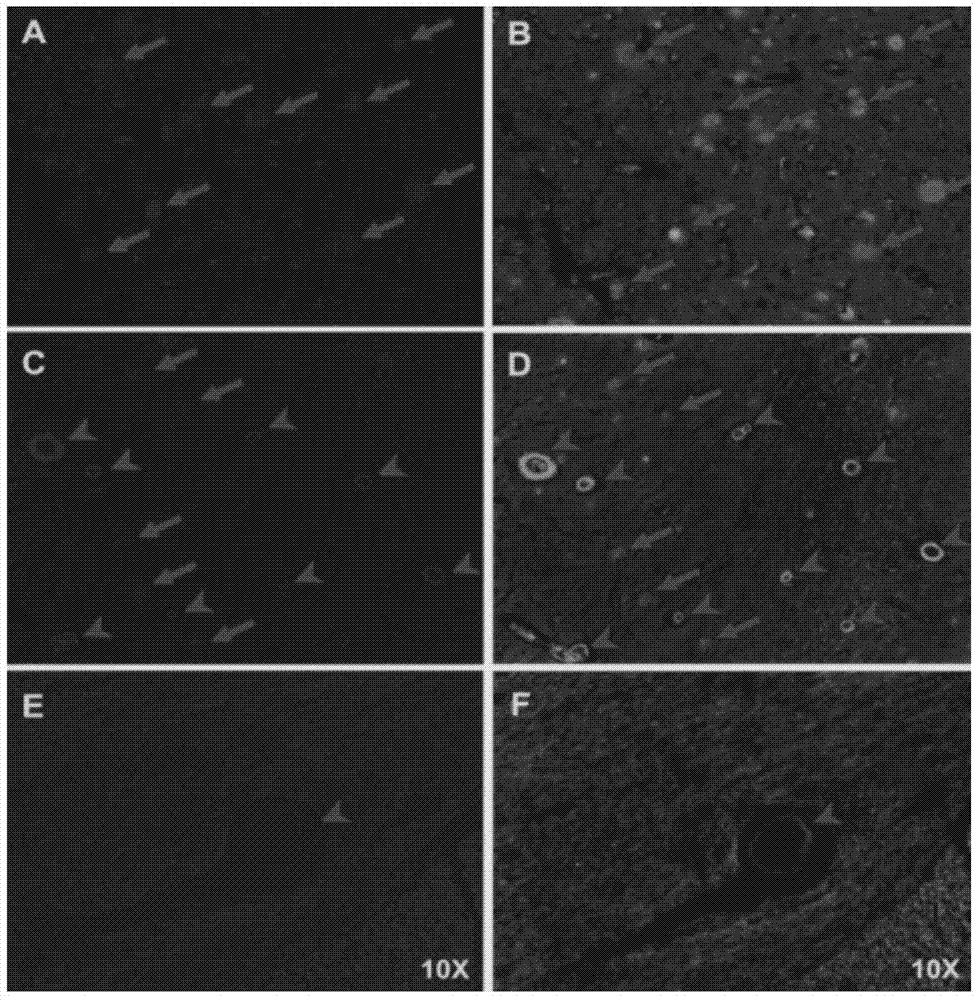
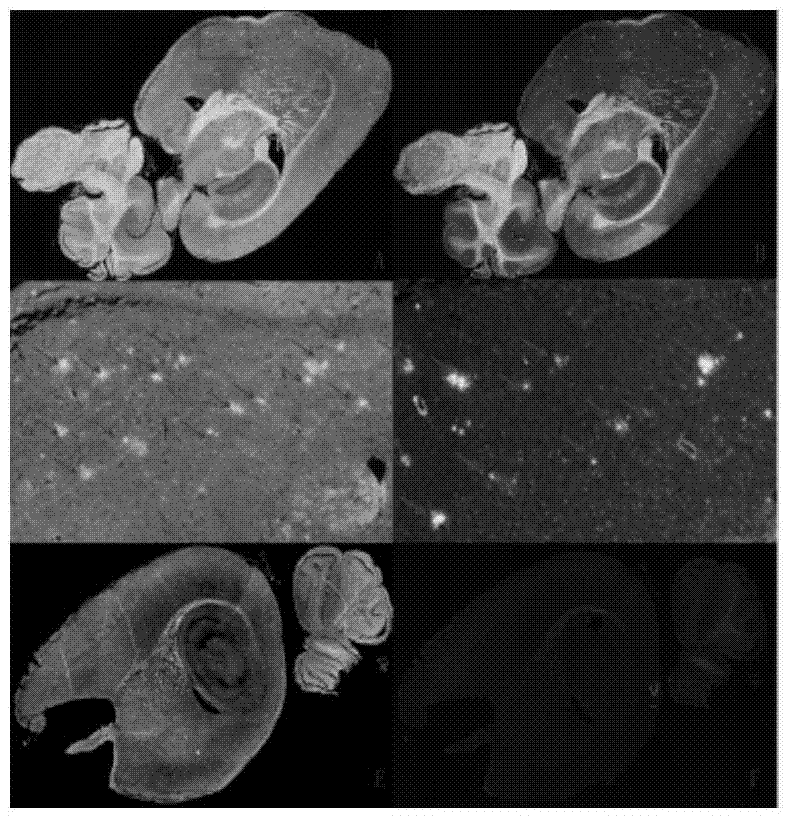
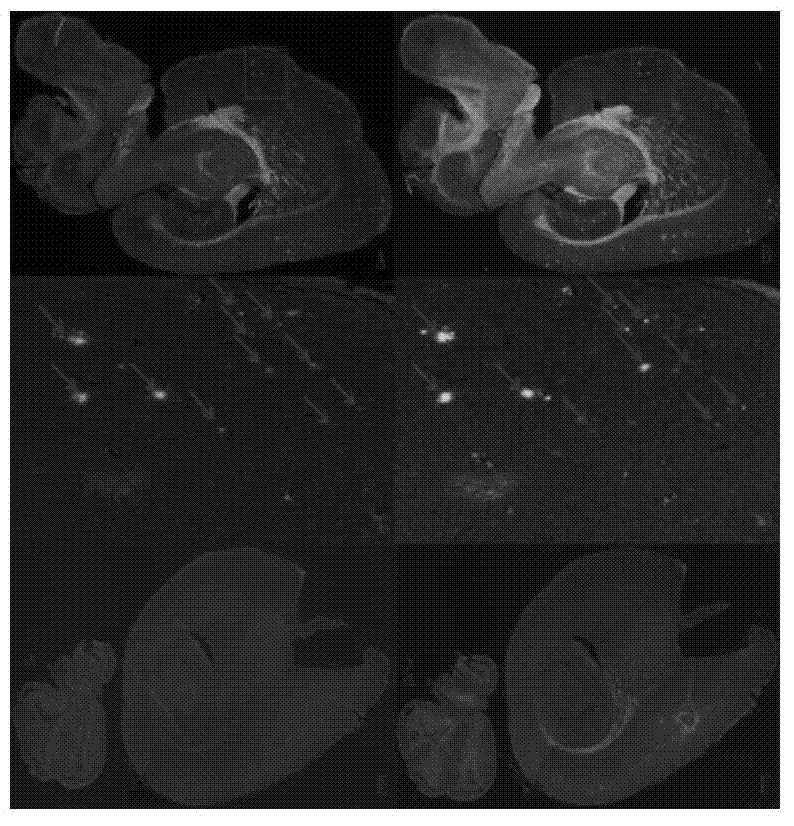
[0153] Example 32-Effect experiment of arylbenzothiazole compounds
[0154] The compounds of the present invention are evaluated for their affinity through competition binding experiments and autoradiography experiments to fully verify their high affinity to Aβ plaques. The initial brain uptake and brain clearance of labeled compounds were evaluated by in vivo biodistribution experiments in normal mice.
[0155] 1. Competitive binding experiments
[0156] Competitive binding experiments (K i Determination): a certain concentration of Aβ 1-42 Aggregate protein with a certain concentration of radioligand [ 125 I] IMPY binding reaction occurs, and the compound to be tested of different concentrations is added simultaneously in the reaction system (respectively compound BT-3-DPA-Re, BT-5-DPA-Re, BT-3-Re, prepared in embodiment 1 and 2 IDA-Re~BT-6-IDA-Re, BTs-3-IDA-Re~BTs-6-IDA-Re) and [ 125 I] IMPY competes with each other, and the complex is separated after equilibrium to ca...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com