Method for preparing chiral alkynyl tertiary alcohol
An alkynyl tertiary alcohol and chirality technology is applied in the field of preparing chiral alkynyl tertiary alcohol, which can solve the problems of inability to large-scale application, low yield, and no natural chirality of abscisic folic acid.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
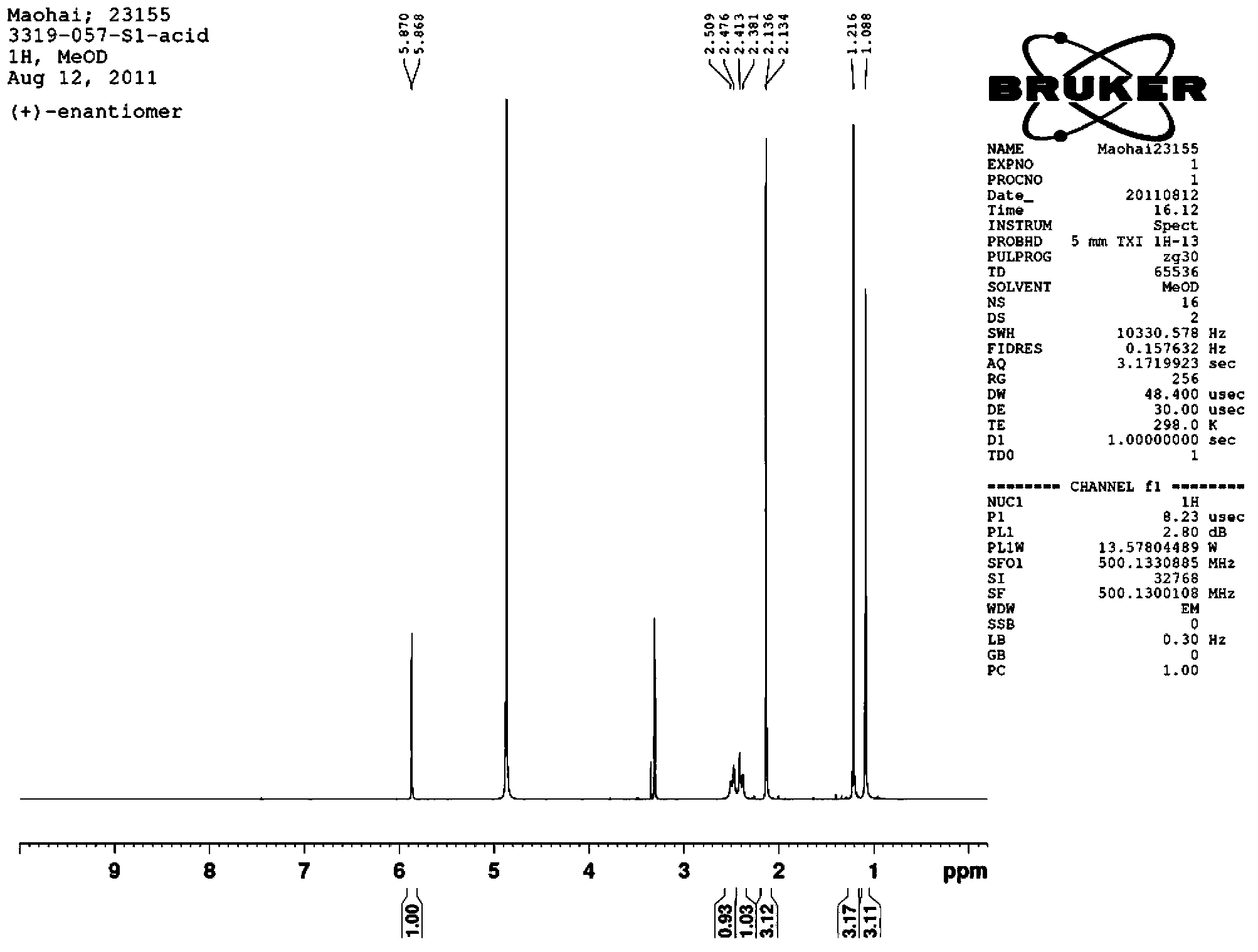
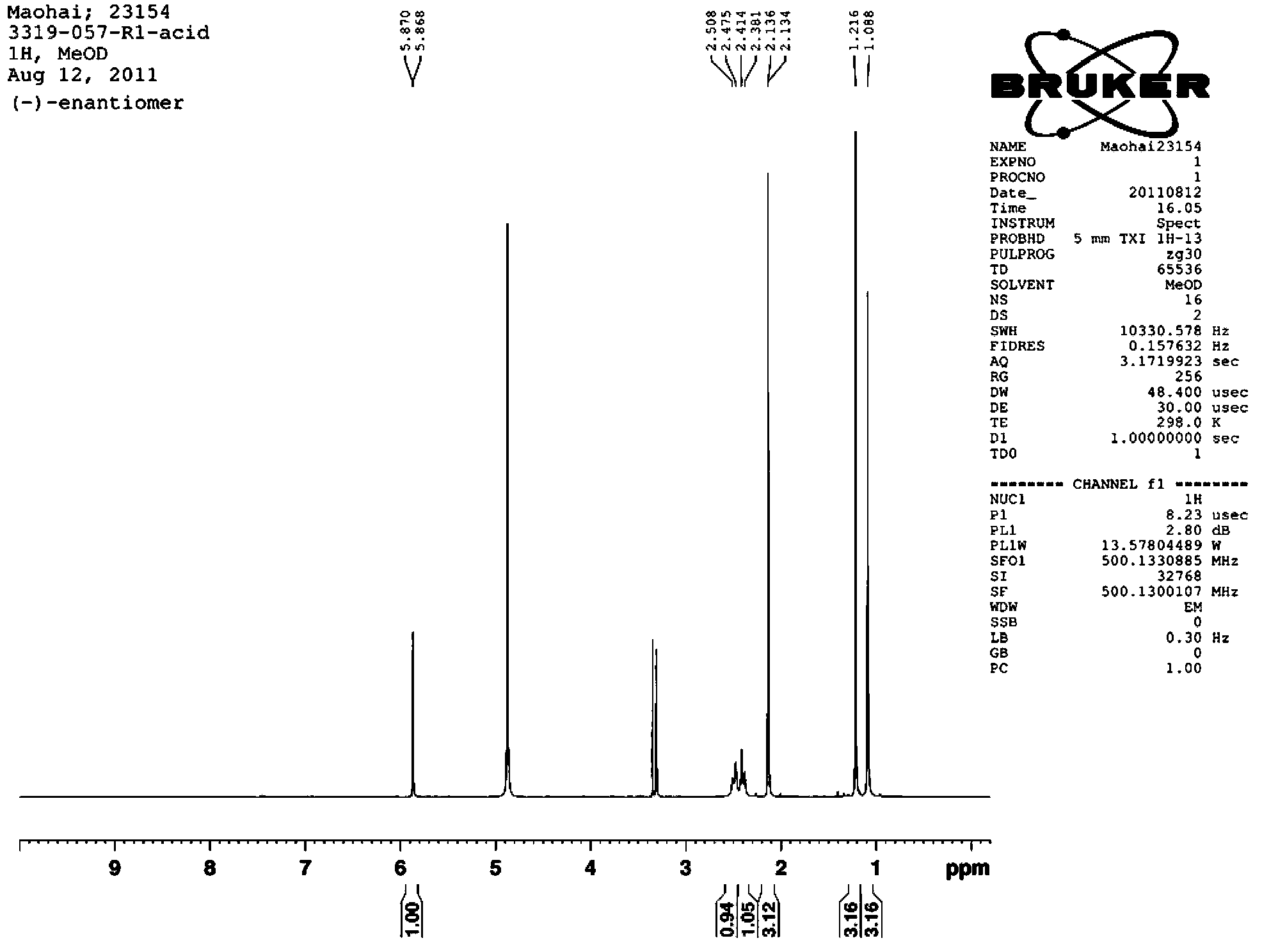
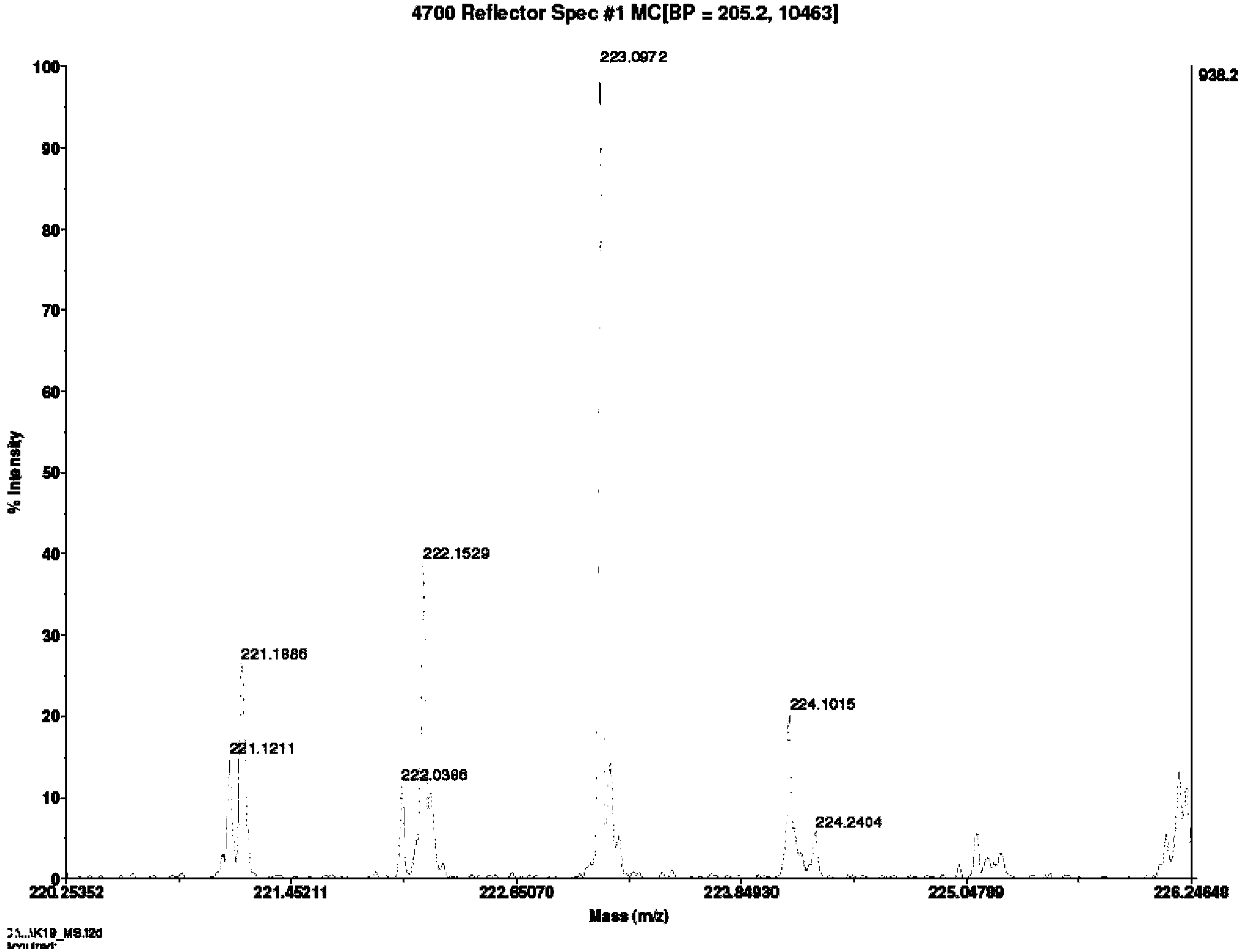
[0023] 1. Synthesis of racemic 4-hydroxy-2-ynyl acid 4:
[0024] Compound 1 can be prepared from 4-oxoisophorone (4-oxoisophorone) according to literature methods, see J.R.Hanson and C.Uyanik, J.Chem.Res., 2003, 7, 426-427. Under nitrogen, 80 mL of dry THF and ethyl propiolate (4.91 g, 50 mmol) were added to a dry 500 mL round bottom flask and magnetic stirring was started. The round bottom flask was immersed in a dry ice acetone bath and cooled until the temperature of the solution in the flask was below -60°C. Lithium diisopropylamide solution (25 mL, 50 mmol, 2 mol / L solution) was slowly added dropwise to the solution in the flask, keeping the internal temperature not exceeding -60 °C. After the dropwise addition, continue to keep stirring at -60°C for one hour.
[0025] Under nitrogen protection, compound 1 (3.92 g, 20 mmol) was dissolved in 20 mL of dry THF, and then slowly added dropwise to the above solution, keeping the temperature not exceeding -60 °C. After the dr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com