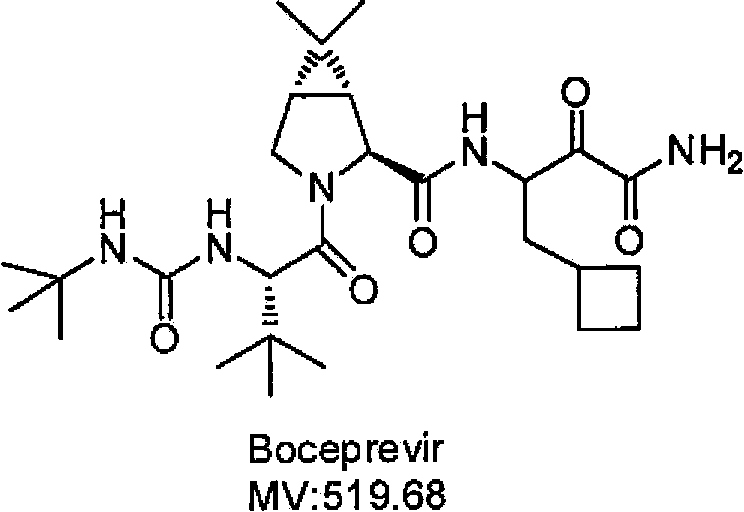
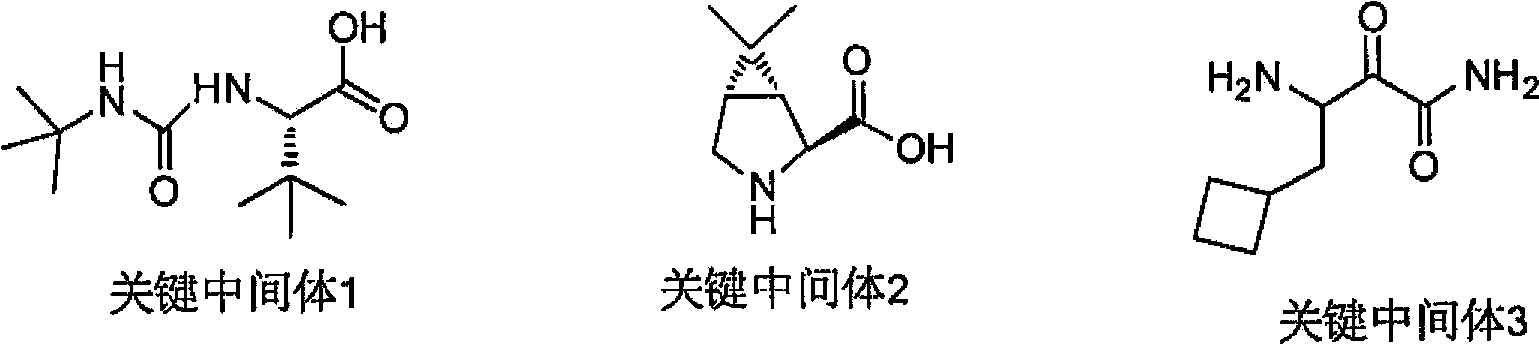
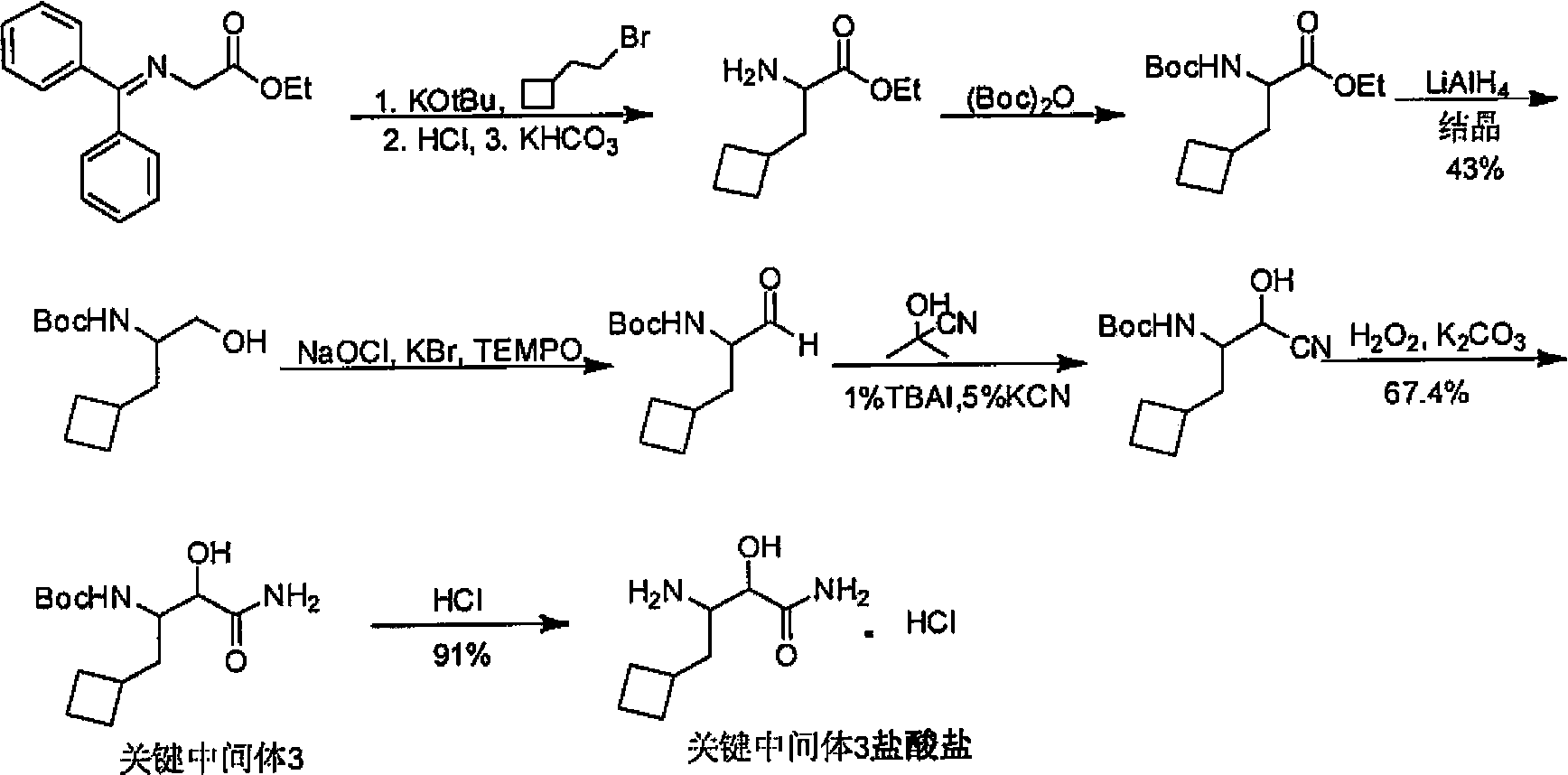
Cyclobutyl-containing alpha-hydroxy-beta-amino ester compound and preparation method thereof
A compound and alkyl technology, applied in the field of α-hydroxy β-amino ester compounds and their preparation, can solve the problems of inability to produce sustained virological response, adverse reactions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0089] Preparation of Compound C:
[0090]
[0091] At 20°C, NaCN (21.2g, 0.433mol) and DMSO140ml were added to the three-necked flask, the temperature was raised to 60°C, bromomethylcyclobutane (42.4g, 0.285mol) was added dropwise, and the reaction solution remained at 70 Stir at -80°C for 3 hours, stop heating, pour the reaction solution into 500ml of ice water, extract the reaction solution with MTBE (250ml*3), combine the organic phase, wash the organic phase with 150ml of water, then add the organic phase to 150ml of 1M HCl After stirring for 30 min, the organic phase was washed with saturated sodium bicarbonate and saturated brine, dried over anhydrous sodium sulfate and evaporated to remove the solvent to obtain 24 g of a slightly yellow liquid, which was the crude compound B, which was directly put into the next reaction without purification.
[0092] N 2 Under protection, compound B (10g, 0.105mol) and 80ml CH 2 Cl 2 Add it into the reaction flask, stir and cool...
Embodiment 2
[0106] where R 1 is benzyl, R 2 Be the preparation of the formula (1) compound of ethyl:
[0107]
[0108] Compound D (0.5g, 2.7mmol) was dissolved in 10ml of phenylacetonitrile, cooled to 0°C, added boron trifluoride diethyl ether (3.6ml, 27.8mmol) dropwise, raised to room temperature and stirred for 4h after the addition, evaporated the solvent under reduced pressure , add 10ml of ethyl acetate to the residue for dilution, add 10ml of saturated sodium bicarbonate and stir for 30min, separate the layers, extract the aqueous phase with ethyl acetate (10ml*3), combine the organic phases and wash with water twice, dry over anhydrous sodium sulfate, After distilling off the solvent, add 20ml of methanol, and add 20ml of 0.5mol / L dilute hydrochloric acid and stir for 90min. After stirring, add saturated sodium bicarbonate solution to adjust the pH to 7, and stir overnight. The liquid was separated and the aqueous phase was extracted with ethyl acetate (20ml*3). The combined o...
Embodiment 3
[0110] where R 1 is phenyl, R 2 Be the preparation of the formula (1) compound of ethyl:
[0111]
[0112] Compound D (0.5g, 2.7mmol) was dissolved in 9ml of benzonitrile, cooled to 0°C, added boron trifluoride diethyl ether (3.6ml, 27.8mmol) dropwise, raised to room temperature and stirred for 4h after the addition, evaporated under reduced pressure Solvent, add 10ml of ethyl acetate to the residue to dilute, add 10ml of saturated sodium bicarbonate and stir for 30min, separate the layers, extract the aqueous phase with ethyl acetate (10ml*3), combine the organic phases and wash with water twice, dry over anhydrous sodium sulfate After evaporating the solvent, add 20ml of methanol, and add 20ml of 0.5mol / L dilute hydrochloric acid and stir for 90min. After stirring, add saturated sodium bicarbonate solution to adjust the pH to 7, and stir overnight. The liquid was separated and the aqueous phase was extracted with ethyl acetate (20ml*3). The combined organic phases were ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com