Insulin-Fc fusion protein
A technology of fusion protein and insulin, which is applied in the field of fusion protein and its preparation, can solve the problems of inconvenient transportation and storage, inability to cure diseases, and short half-life of storage, etc., to improve pharmacokinetic performance, reduce medication pain, and reduce intervals the effect of time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
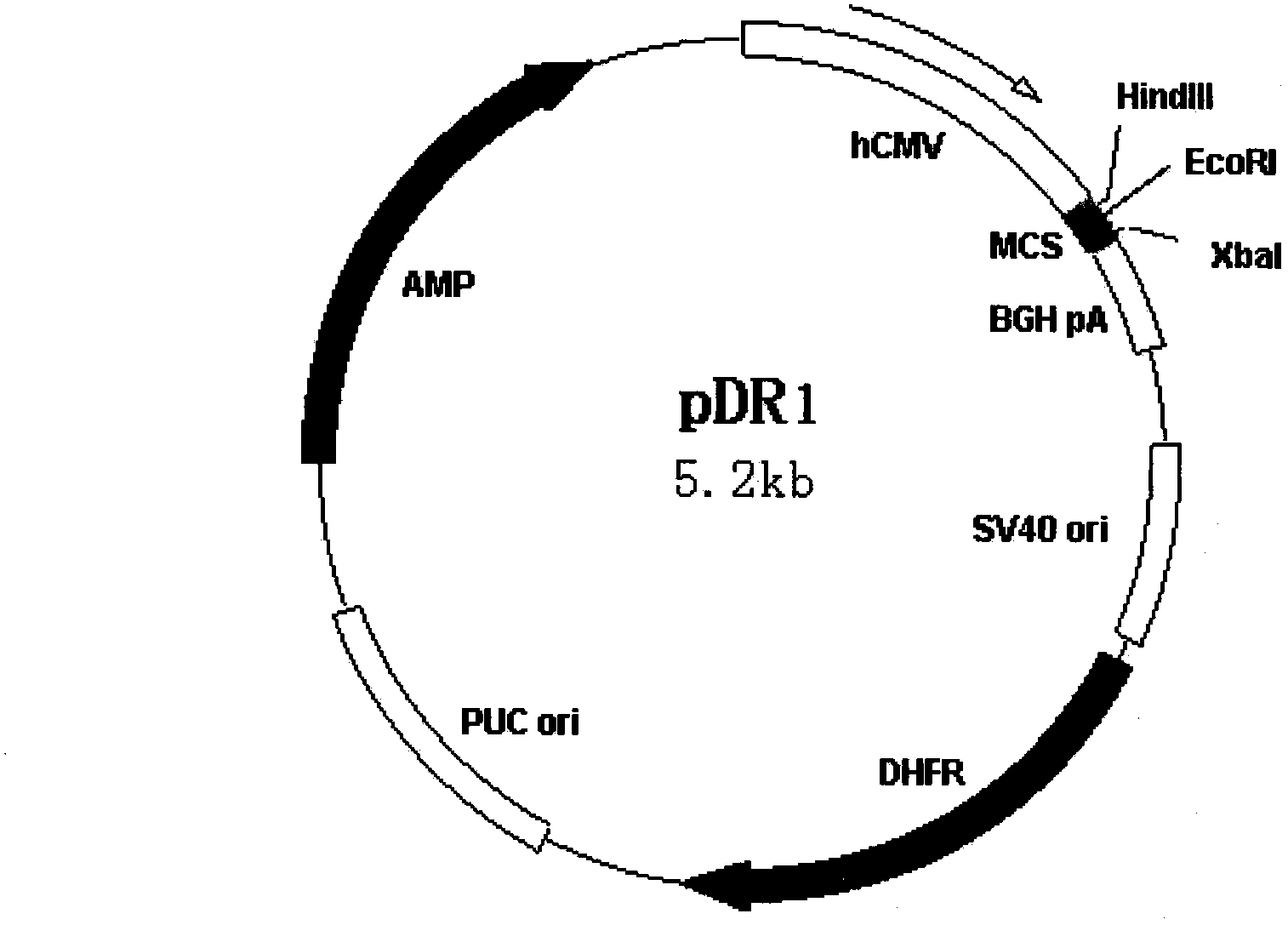
[0023] Example 1: Construction and expression of insulin-Fc fusion protein
[0024] Isolate healthy human lymphocytes with lymphocyte separation fluid, extract total RNA with Trizol reagent (Invitrogen company product), according to literature (Cell, 1980, 22:197-207) and literature (Nucleic Acids Research, 1982, 10: 4071-4079 ) respectively designed primers FCM SENSE: GAGCCCAAATCTTGTGACAAAACTCACACATGCCCACCGTGCCCAGCACCTGAAGCAGCAGGGGGACCGTCAGTCTTCC and FCANTISENSE: CGAATTCTCATTTACCCGGAGACAGG to amplify the Fc region of the antibody, and introduced mutation points into FCM SENSE to mutate the two leucines of H235 and H236 into two alanines. All the reactions were hot-started, and the reaction conditions were: 94°C for 5 minutes; 94°C for 45 seconds, 60°C for 45 seconds, 72°C for 1 minute and 10 seconds, 30 cycles; 72°C for 10 minutes. Insulin B chain, C is 1, GGGPGKR, 2, GGGPQT, 3, GGGPGAG, 4, AAGGGPSVR) and A chain genes were synthesized from the whole gene, and connected to T...
Embodiment 2
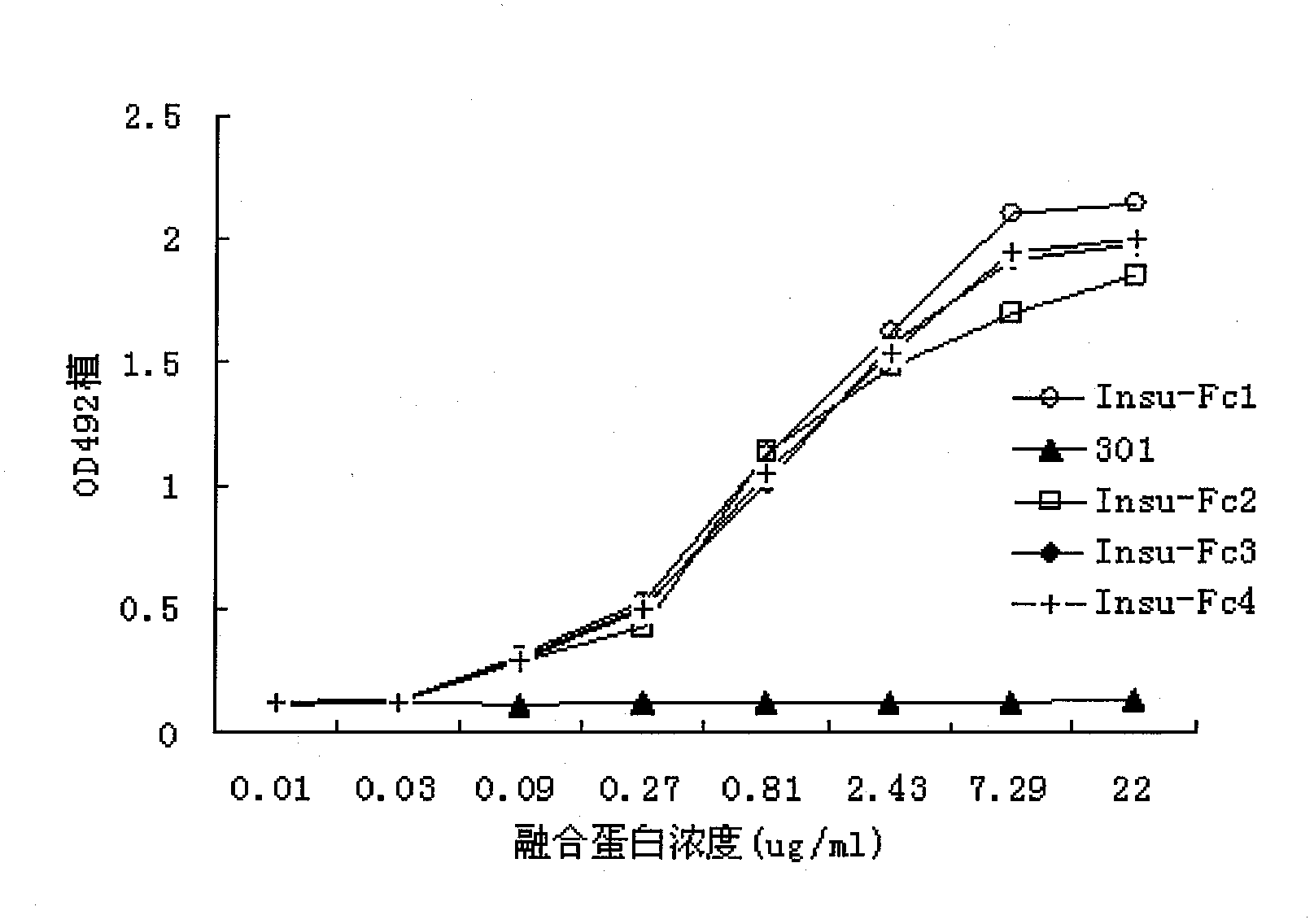
[0026] Example 2: Detection of insulin-Fc fusion protein affinity
[0027] ELISA identifies the binding activity of insulin-Fc fusion protein: the soluble insulin receptor protein (Abnova company product) is diluted to 2ug / ml with 0.05mmol / L sodium carbonate.Sodium bicarbonate buffer solution (pH 9.6), 100ul / hole, 4 °C overnight. After blocking with 3% skimmed milk at room temperature for 2 hours, different concentrations of insulin-Fc fusion protein and control antibody 301 were added, 100ul / well, and 3 parallel wells were taken for each concentration, and incubated at room temperature for 2 hours. Discard the supernatant, wash with PBS three times, add HRP-labeled mouse anti-human IgG monoclonal antibody (product of DAKO Company) diluted according to the titer, 50 μl / well, and incubate at room temperature for 45 minutes. After fully washed with PBS, the OPD was protected from light for color development, and 2mol / L H2SO4 was added to terminate the reaction, and the absorba...
Embodiment 3
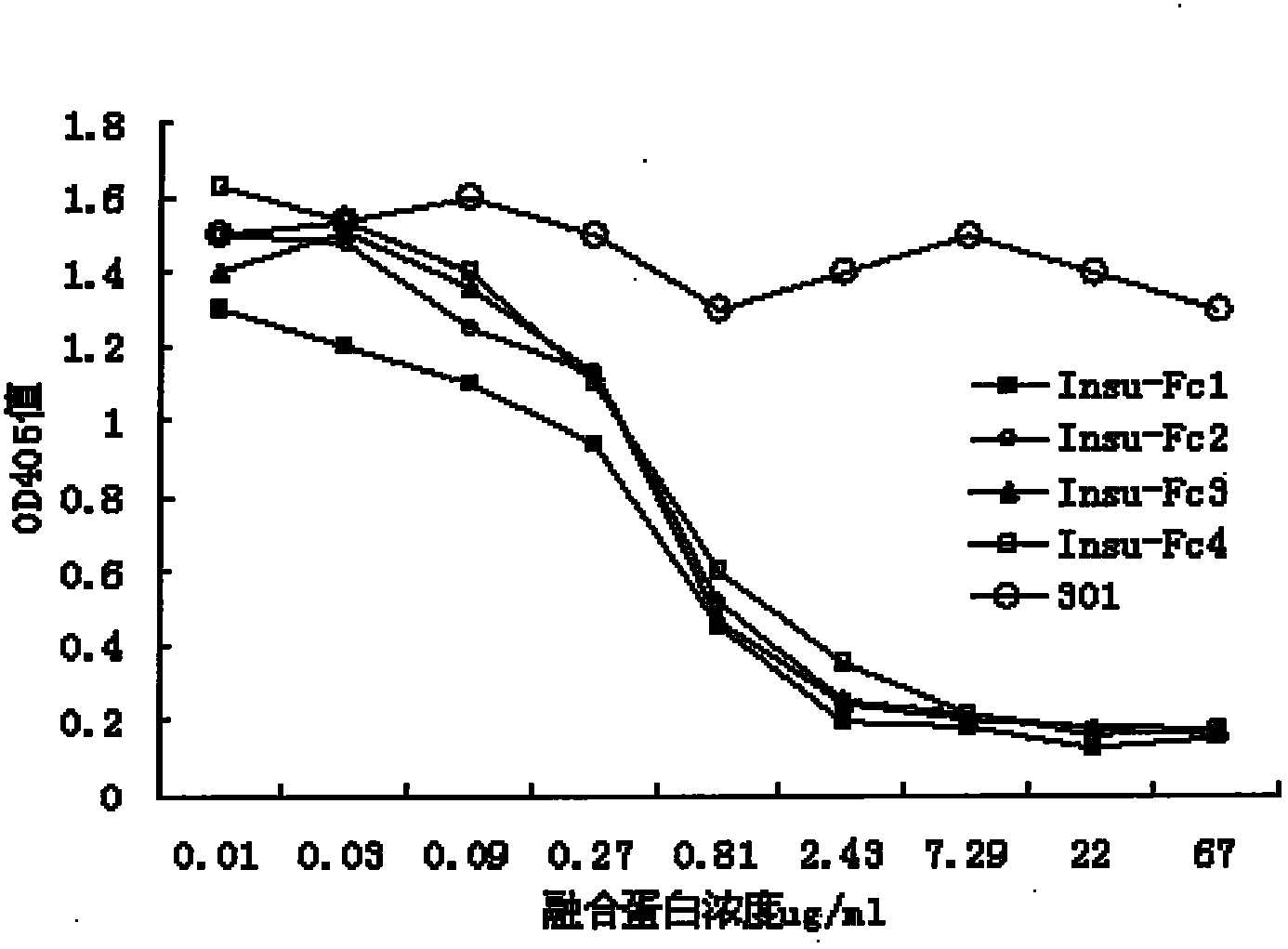
[0029] Embodiment 3: Detection of the affinity constant of insulin-Fc fusion protein with biocore
[0030] Biacore T100 was used to detect the binding activity of each insulin-Fc fusion protein to insulin receptor. The insulin receptor was connected to the CM5 chip by the method of amino coupling, and then the insulin receptor and The binding activity of each insulin-Fc fusion protein, the flow rate of the flow cell is 30ul / min. The insulin receptor is coupled to a response value of 1500RU, and insulin and each insulin-Fc fusion protein (50ug / ml, 25ug / ml, 12.5ug / ml, 6.25ug / ml, 3.125ug / ml, 1.56ug / ml, 0.78ug / ml, 0.39ug / ml) the signal activity of interacting with the insulin receptor at different concentrations. The binding time was 420 s, the dissociation time was 600 s, and the regeneration time was 60 s with 10 mM glycine. The baseline was stable, and 1.56 ug / ml was used as the repeated concentration to ensure the regeneration activity of the chip. According to the calculat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com