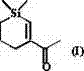Silica hybrid dehydroherbac and preparing method thereof
A technology of dehydroherbone and sila, which is applied in the compound field of sila dehydroherbone, and achieves the effects of wide application range, simple and easy operation of experimental equipment and operation, and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Add 1 mol of 1,1-dimethylsilacyclobutane II, 1 mol of 3-butyn-2-one III, 1% equivalent of Pd(PPh 3 ) 4 and 5 L of benzene solvent, heated to 80°C, and reacted for 4 hours with magnetic stirring. Concentrate after the reaction, decolorize and separate on a silica gel column, use petroleum ether: ether=10:1 mixed solvent as eluent, obtain pure product sila dehydroherbone I (purity > 98%, colorless liquid), and the isolated yield 50%. The NMR and high resolution mass spectrometry data of this compound are as follows: 1 H NMR (CDCl 3 ) δ = 6.80 (s, 1H), 2.34-2.31 (m, 5H), 1.81-1.76 (m, 2H), 0.69-0.66 (m, 2H), 0.13 (s, 6H); 13 C NMR (CDCl 3 ) δ = 200.35, 156.47, 138.19, 27.75, 25.32, 20.95, 11.14, -2.30. HRMS: m / z : calcd for C 9 h 16 OSi [M+H] + : 169.1443, found 169.1449.
Embodiment 2
[0029] Add 2 mol of 1,1-dimethylsilacyclobutane II, 1 mol of 3-butyn-2-one III, 5% equivalent of (PPh 3 ) 2 PdCl 2 and 2 L of benzene solvent, heated to boiling, and reacted with magnetic stirring for 2 hours. Concentrate after the reaction, decolorize and separate on a silica gel column, and use a mixed solvent of petroleum ether: ether = 10:1 as the eluent to obtain the pure product sila dehydroherbalone I with an isolated yield of 65%. See Example 1 for NMR and high-resolution mass spectrometry data.
[0030] The reaction formula of embodiment 1 and embodiment 2 is as follows:
[0031]
[0032] Synthesis of siladehydroherbone I by two-step reaction
Embodiment 3
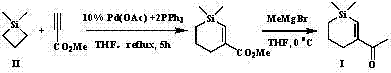
[0034] Into a 10 L round bottom flask, add 1 mol of 1,1-dimethylsilacyclobutane II, 1 mol of methyl propiolate, 10% equivalent of Pd(OAc) 2 +2PPh 3 and 5 L of tetrahydrofuran solvent, heated to boiling, and reacted with magnetic stirring for 5 hours. Concentrate after the reaction, decolorize and separate on a silica gel column, use a mixed solvent of petroleum ether: ethyl ether = 5:1 as an eluent to obtain pure product 1,1-dimethyl-3-methoxycarbonyl-silacyclohexene, and analyze The data can be found in the literature ( Bull. Chem. Soc. Jpn. , 1991, 64, 1461).
[0035] Add 10 mmol of 1,1-dimethyl-3-methoxycarbonyl-silacyclohexene and 150 mL of THF to a 250 mL round bottom flask, then cool to 0 °C with an ice bath, and , slowly dropwise added 10 mmol of MeMgBr for 1 hour. The reaction was quenched with 1 M dilute hydrochloric acid solution, the resulting solution was extracted three times with diethyl ether, the solvent was removed by rotary evaporation, decolorized and s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com