Silica hybrid dehydroherbac and preparing method thereof
A technology of herbal ketones and silicon impurities, applied in the field of compounds and their general synthesis, to achieve the effects of easy-to-obtain raw materials, high separation yield, simple experimental equipment and operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
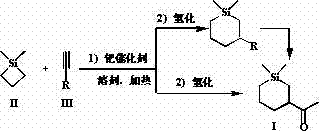
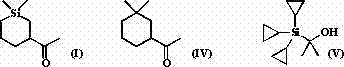
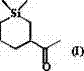
[0028] Add 1 mol of 1,1-dimethylsilacyclobutane II, 1 mol of 3-butyn-2-one III, 10% equivalent of (PPh 3 ) 2 PdCl 2 and 5 L of benzene solvent, heated to 80°C, and reacted with magnetic stirring for 4 hours. Cool the reactant to room temperature, add 10% equivalent Ru / C, maintain H 2 Pressure and normal pressure, after 2 hours after the reaction, concentrated to remove the solvent, decolorized and separated on a silica gel column, and used a mixed solvent of petroleum ether: ether=10:1 as an eluent to obtain the pure product Sizadone I (purity > 98%, Colorless liquid), isolated yield 60%. The NMR and high resolution mass spectrometry data of this compound are as follows: 1 H NMR (CDCl 3 ) δ = 2.45-2.38 (m, 1H), 2.12 (s, 3H), 2.06-2.02 (m, 1H), l.85-1.82 (m, 1H), 1.46-1.40 (m, 1H), 1.19- 1.11 (m, 1H), 0.89-0.86 (m, 1H), 0.74-0.71 (m, 1H), 0.60-0.54 (m, 1H), 0.47-0.41 (m, 1H), 0.07 (m, 3H), 0.03 (m, 3H). 13 C NMR (CDCl 3 ) δ 212.92, 49.79, 31.24, 26.91, 23.12, 16.88, 13...
Embodiment 2
[0030] Into a 5 L round bottom flask, add 1.5 mol of 1,1-dimethylsilacyclobutane II, 1 mol of 3-butyn-2-one III, 5% equivalent of Pd(PPh 3 ) 4 and 2 L of toluene solvent, heated to 100°C, and reacted with magnetic stirring for 2 hours. Cool the reactant to room temperature, add 10% equivalent of Pd / C, maintain H 2 Pressure and normal pressure, after 1 hour after the reaction, concentrate to remove the solvent, decolorize and separate on a silica gel column, use a mixed solvent of petroleum ether: ethyl ether = 10:1 as the eluent, and obtain the pure product Sizadone I, with an isolation yield of 56% . See Example 1 for NMR and high-resolution mass spectrometry data.
[0031] The reaction formula of embodiment 1 and embodiment 2 is as follows:
[0032]
[0033] Synthesis of Sizaherdone I by Two-step Reaction
Embodiment 3
[0035] Into a 10 L round bottom flask, add 1 mol of 1,1-dimethylsilacyclobutane II, 1 mol of methyl propiolate, 10% equivalent of Pd(OAc) 2 +2PPh 3 and 5 L of tetrahydrofuran solvent, heated to boiling, and reacted with magnetic stirring for 5 hours. Concentrate after the reaction, decolorize and separate on a silica gel column, use a mixed solvent of petroleum ether: ethyl ether = 5:1 as an eluent to obtain pure product 1,1-dimethyl-3-methoxycarbonyl-silacyclohexene, and analyze The data can be found in the literature ( Bull. Chem. Soc. Jpn. , 1991, 64, 1461).
[0036] Add 10 mmol of 1,1-dimethyl-3-methoxycarbonyl-silacyclohexene and 150 mL of THF to a 250 mL round bottom flask, add 10% equivalent Ni / C, and maintain H 2 Normal pressure, 1 hour after reaction. Then it was cooled to 0° C. with an ice bath, and 10 mmol of MeMgBr was slowly added dropwise under magnetic stirring for 1 hour. The reaction was quenched with 1 M dilute hydrochloric acid solution, the resulting ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com