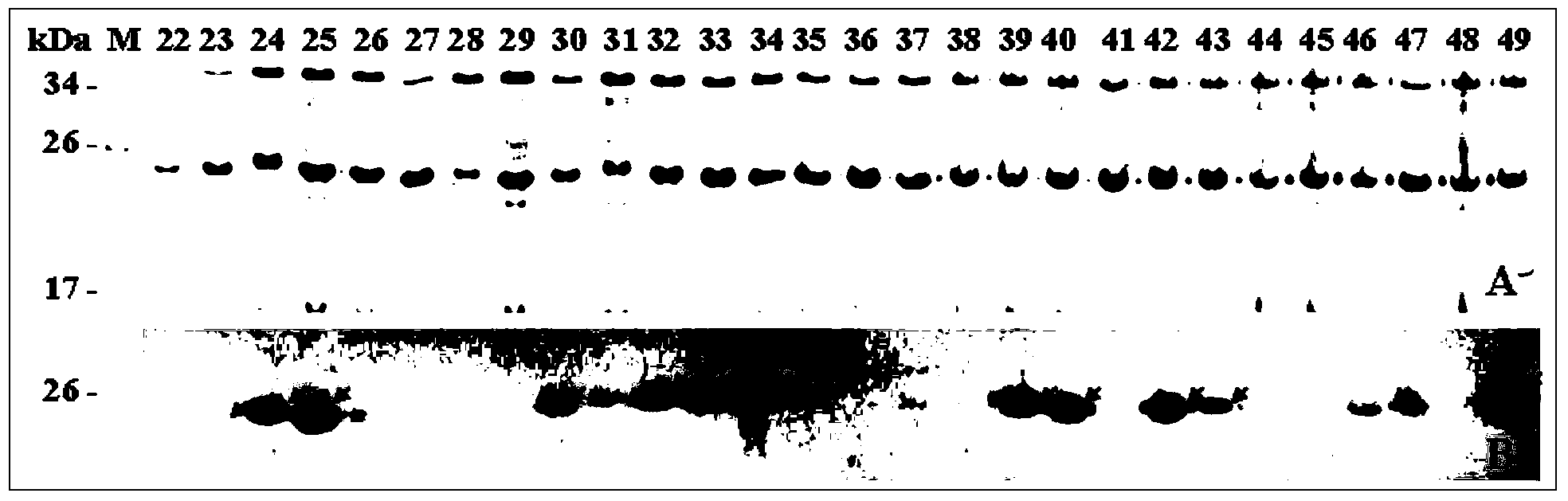Epitope peptide in human spawn ZP (zona pellucida) protein 4 and application of epitope peptide
A technology of zona pellucida protein and protein, applied in the field of epitope peptides, which can solve the problems of widely used diagnostic methods that affect the accuracy of clinical diagnosis, easy to produce non-specific cross-reactions, false positives, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0102] Embodiment 1: Preparation of rabbit anti-human ZP4C antiserum
[0103] Materials and methods:
[0104] 1. Human ZP4 gene clone was donated by Dr. Jurrien Dean, Laboratory of Cell and Developmental Biology, National Institutes of Health. For the construction of heat-inducible expression plasmids pSY621 and pXXGST-1, see Shen Yun, Ying Kang, Xu Wanxiang, Xie Yi: Construction of high-efficiency expression vector pSY621 in Escherichia coli. Fudan Journal (Natural Science Edition) 2000; 39(3): 313– 317; Xu WX, et al. Minimal motif mapping of a known epitope on human zona pellucida protein-4 using a peptide biosynthesis strategy. J Reprod Immunol 2009;81:9-16. Escherichia coli BL21 (DE3) strain was preserved by the State Key Laboratory of Genetic Engineering, Fudan University.
[0105] 2. For the preparation of mouse anti-huZP4 monoclonal antibody MA-1662, see Bukovsky A, Gupta SK, et al. Production of monoclonal antibodies against recombinant human zona pellucida glycoprot...
Embodiment 2
[0113]Example 2 The first round of epitope scanning mapping with the target protein of 18 peptides
[0114] Material:
[0115] 1. For the relevant experimental materials, see the part 1 of the embodiment
[0116] 2. The two ends are BamH I and Sal I cohesive ends respectively, and the positive and negative strand DNA fragments with 18 peptide coding DNA sequences plus TAA stop codon in the middle are synthesized by Shanghai Jierui Bioengineering Co., Ltd.
[0117] 3. The series of 18 peptide-encoding DNA sequences are based on the public information of the human ZP4 protein-encoding gene and protein amino acid sequence (Harris JD, et al. Cloning and characterization of zona pellucida genes and cDNAs from a variety of mammalian species: the ZPA, ZPB and ZPC gene families. DNA Seq 1994;4:361-393).
[0118] Method (identification of antigenic (reactive) 18 peptide (fusion protein)):
[0119] 1. After using the rabbit anti-recombinant human ZP4C antiserum to verify the immunobl...
Embodiment 3
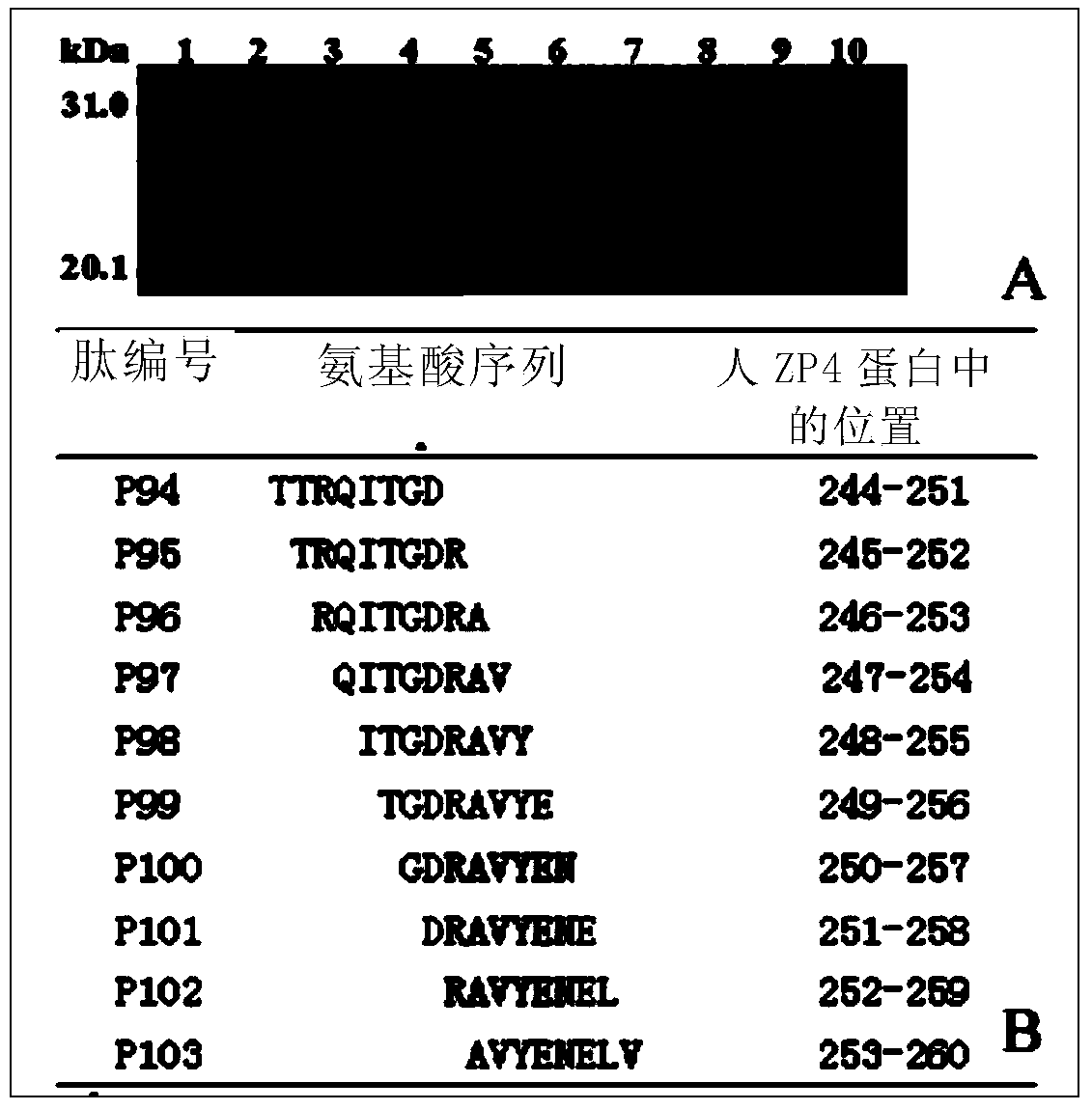
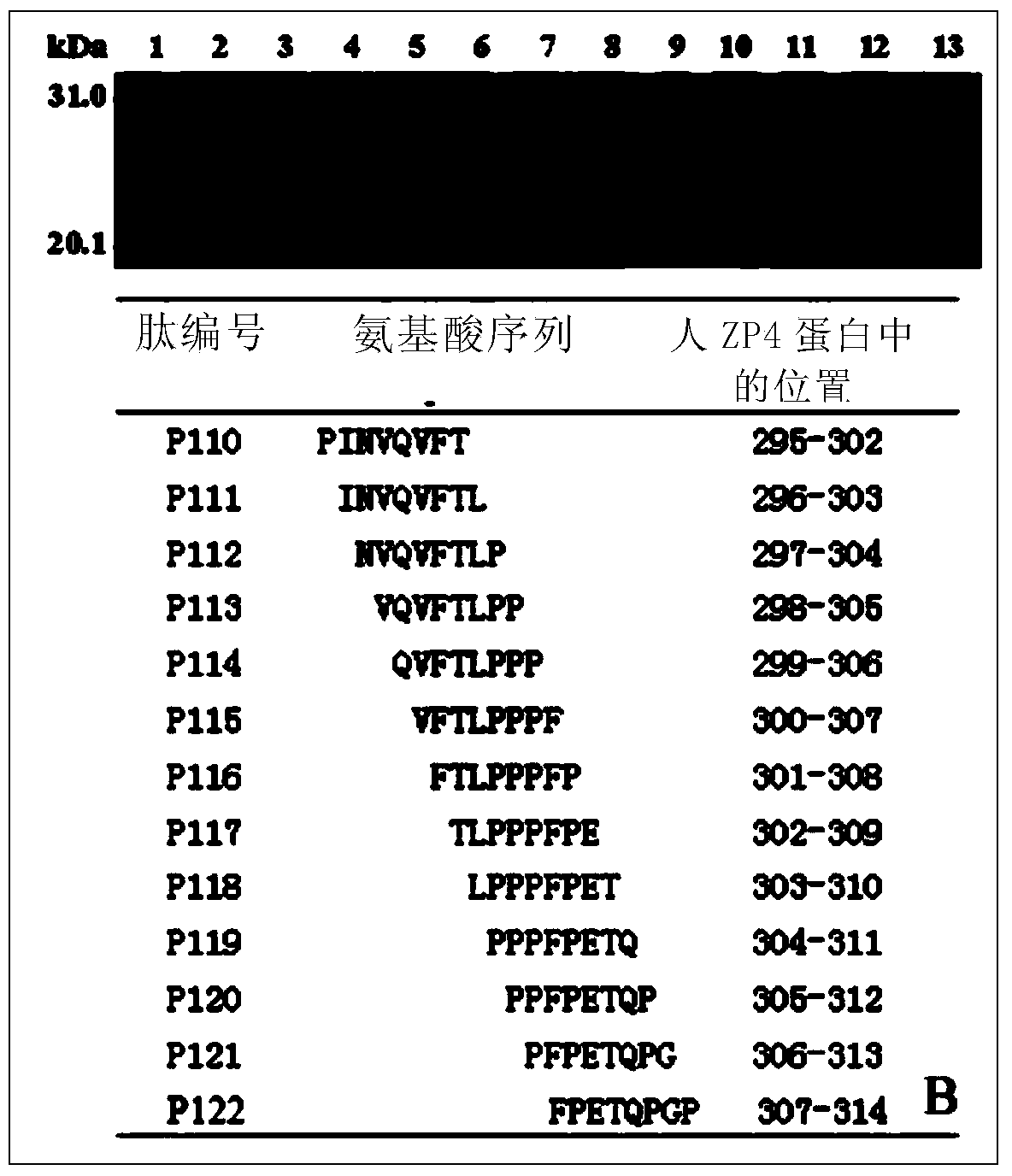
[0125] Example 3 Identification of Two Fine Epitope Peptides in Human ZP4C
[0126] Material
[0127] 1. For the relevant experimental materials, see the part 1 of the embodiment
[0128] 2. The two ends are BamH I and Sal I cohesive ends respectively, and the positive and negative strand DNA fragments in the middle are each 8-peptide coding DNA sequence plus TAA stop codon, which were synthesized by Shanghai Jierui Bioengineering Co., Ltd.
[0129] method
[0130] 1. According to the two adjacent reactive 18 peptides of P30-P31 and P33-P34 in Example 2, design a series of 8 peptides (numbering P110-P122 and P134-P146) covering their full-length sequence overlapping 7 amino acid residues , image 3 Only P134-P141 is shown in B) encoding DNA positive and negative strand fragments (positive strand 5'-end plus 5'-gatcc, 3'-end plus taag-3'; negative strand 5'-end plus 5'-tcgactta, 3 '-end plus g-3'), send DNA chemical synthesis;
[0131] 2-5. The identification steps from se...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com