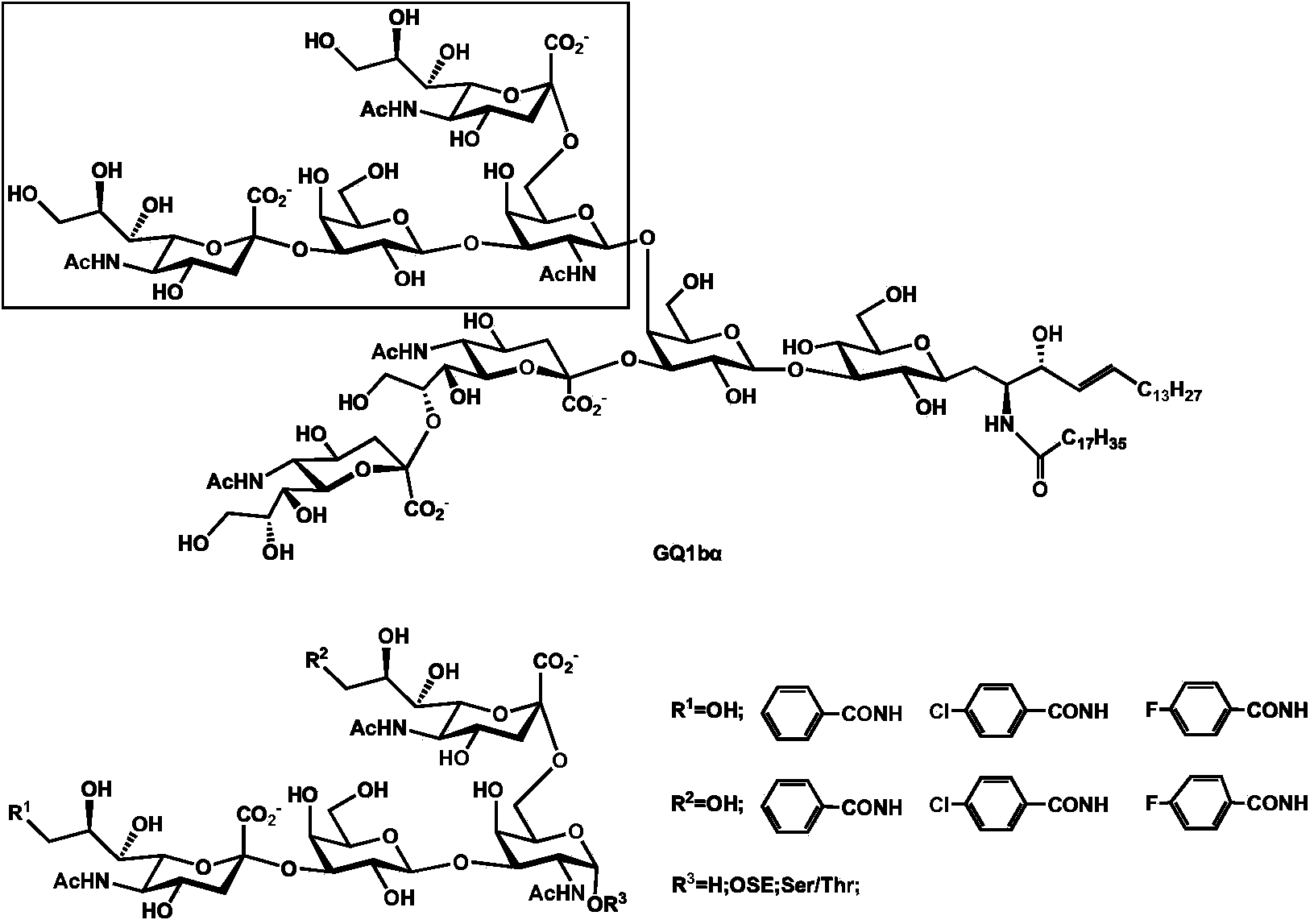
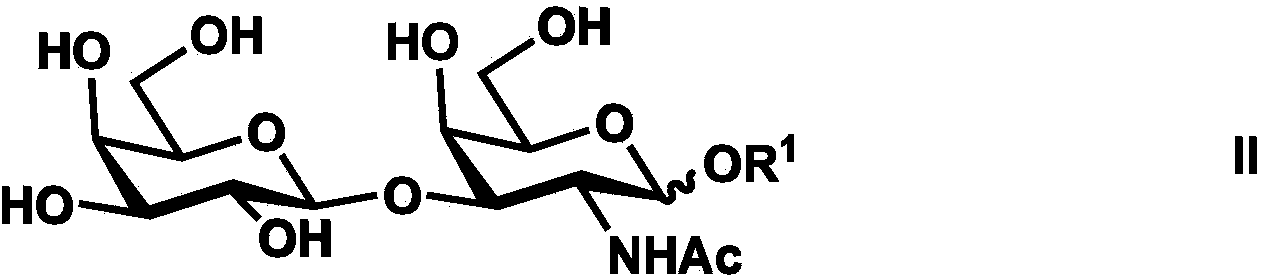
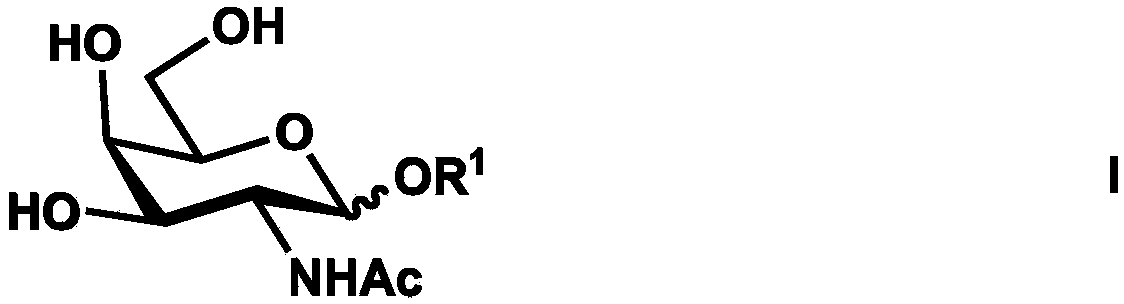Synthesis method of tetrasaccharide MAG antagonist
A synthesis method and antagonist technology, applied in the field of carbohydrate drugs, can solve the problems of narrow substrate applicability, difficulty in expression, and difficulty in obtaining soluble protein, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Embodiment 1 Chemoenzymatic Synthesis of Tetrasaccharide MAG Receptor Antagonist
[0051] Proceed as follows:
[0052] (1) Chemical synthesis of β-configuration monosaccharide compound 1
[0053] Compound 1 (GalNAcβProN 3 )Synthesis
[0054] The reaction equation is as Figure 8 shown;
[0055] Add galactosamine hydrochloride (8.0g, 37.1mmol), acetic anhydride (38mL), pyridine (160mL), dimethylaminopyridine (DMAP, 0.3g, 2.46mmol) into a 500mL round bottom flask, and stir at room temperature for 12 hours . Thin-layer chromatography detection (EA:MeOH=10:1) After the reaction is complete, concentrate by rotary evaporation, then add 20 mL of toluene to the reaction solution, concentrate by rotary evaporation, and repeat 3 times. The obtained solid was redissolved in 300 mL of methanol, and left to stand at 4° C. for 12 hours for recrystallization. After filtration, the filtrate was discarded, and the resulting solid was collected and dried to obtain compound 7 (11.7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com