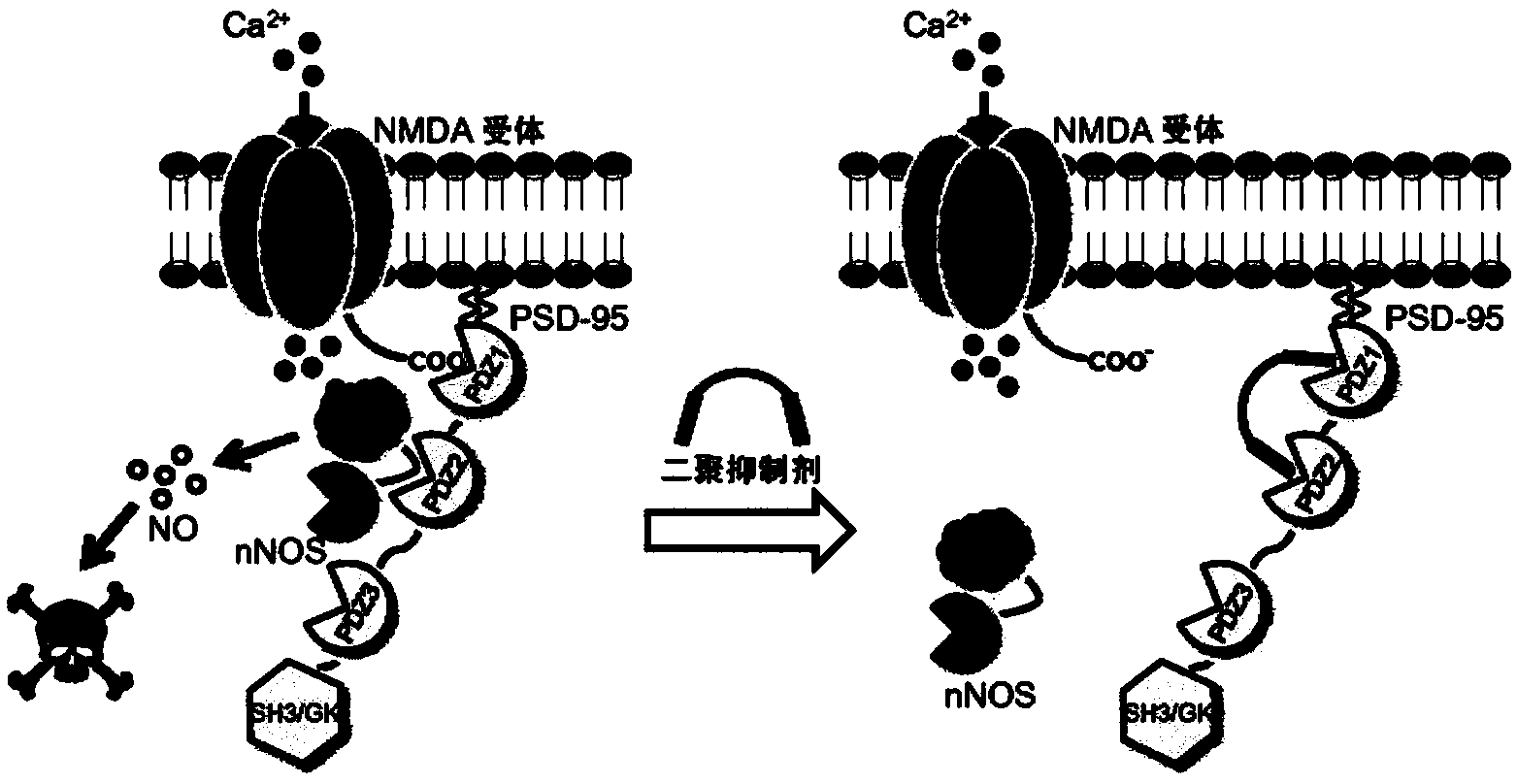
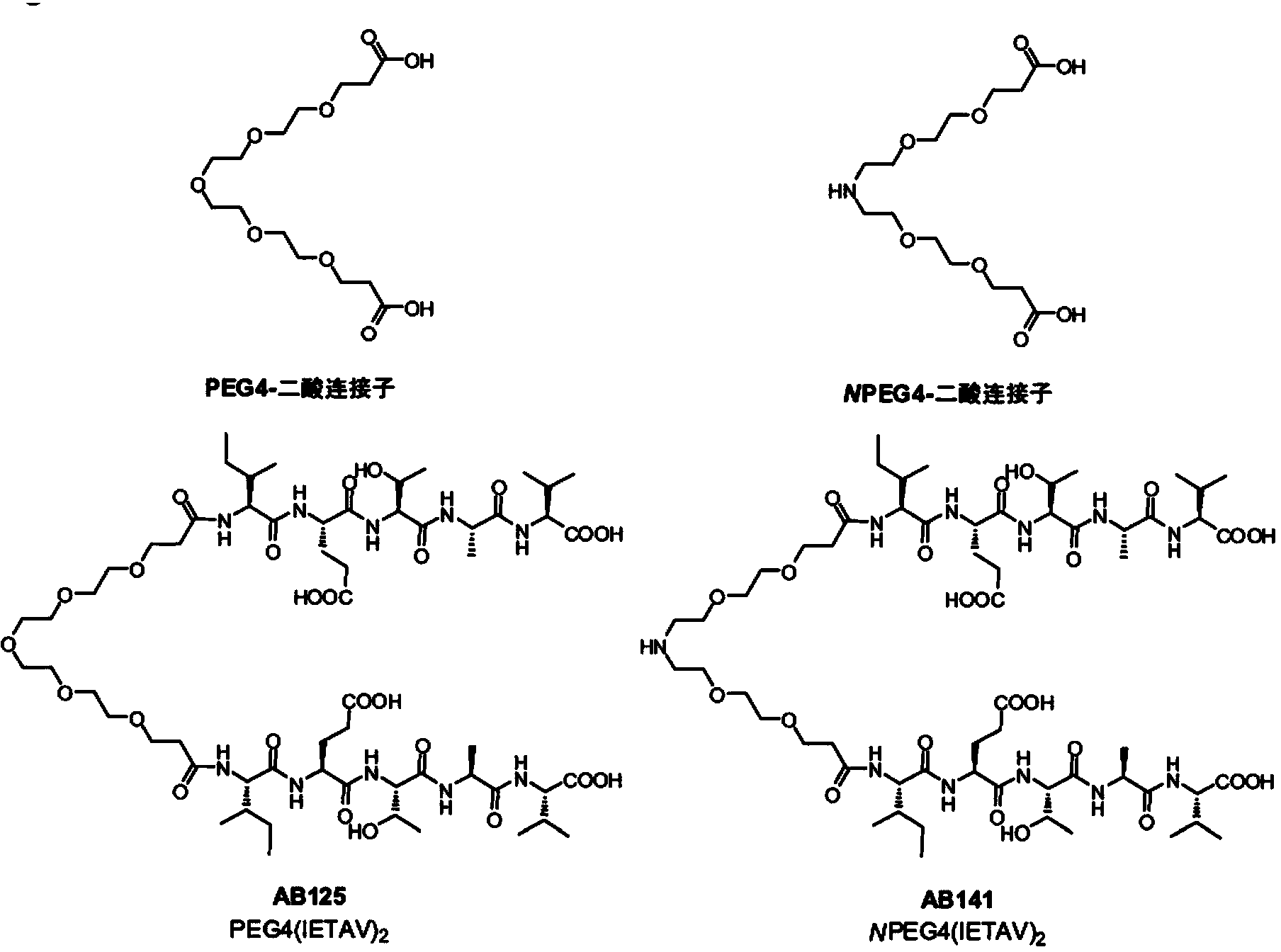
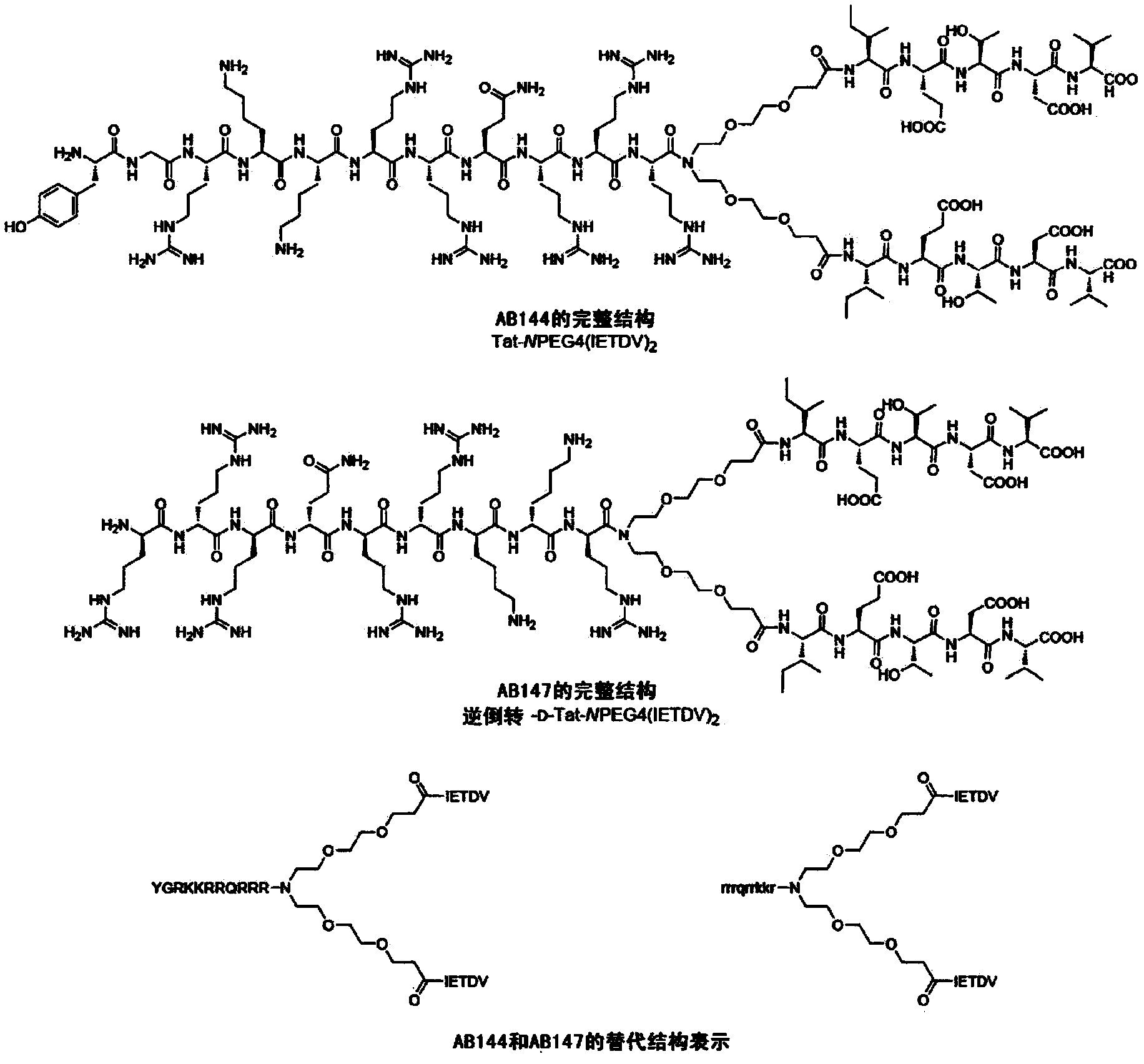
High-affinity, dimeric inhibitors of psd-95 as efficient neuroprotectants against ischemic brain damage and for treatment of pain
A diacid and ethylene glycol technology, applied in the field of dimer peptide analogs, can solve the problem of side effects and cannot be used clinically
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0111] According to a first embodiment, the dimeric PSD-95 inhibitor comprises a CPP linked directly or indirectly via a chemical bond to a nitrogen atom in the backbone of the NPEG linker in the inhibitor, wherein the nitrogen atom is symmetrically or asymmetrically located in the linker. The attachment of CPP to the nitrogen atom in the NPEG linker can be mediated via an amide bond, a maleimide coupling, a disulfide bond, or an amino-reactive electrophile selected from the group consisting of N-hydroxysuccinyl Amine (NHS) ester, p-nitrophenyl ester, succinimidyl carbonate, p-nitrophenyl carbonate, succinimidyl polyurethane, isocyanate, isothiocyanate, acyl azide, sulfuryl chloride, Aldehydes, carbonates, imidates or anhydrides, and thio-reactive groups selected from the group consisting of haloacetyls, haloalkane derivatives, aziridines, acryloyl derivatives arylating agents.
[0112] Alternatively, the attachment of the CPP to the nitrogen atom of the linker may be mediated...
Embodiment 1
[0165] Example 1. Synthesis of Dimeric PSD-95 Inhibitors
[0166] 1.1 Synthesis of Ns-NPEG4-diacid-linker A-C (Scheme 1- Figure 16 )
[0167] For the synthesis of Ns-NPEG4-diacid-linker A (3; Scheme 1), 2-chlorotrityl chloride resin (3 mmol, 1.90 g) was rinsed and swelled in DMF (20 min). Fmoc-NH-PEG was synthesized by adding 1 (2mmol, 800mg) in DMF (8mL) to the drained resin followed by DIPEA (10mmol, 1.75mL) 2 -CH 2 CH 2 COOH (1, Protocol 1; Biomatrik Inc., Jiaxing, China) was loaded onto the resin. After shaking for 60 minutes, methanol (1 mL, 25 mmol) was added and shaking was continued for another 5 minutes. Allow the loaded resin to drain, then rinse thoroughly with DMF (10-15 flowing washes, 10 ml each), deprotect the Fmoc group with 20% piperidine in DMF for 5 minutes, rinse with DMF in between, and then use DMF and THF rinse for 15 minutes. The resin was swelled in DIPEA (12 mmol, 2.1 mL) and THF (8 mL) for 15 min, then o-nitrobenzenesulfonyl chloride (NsCl,...
Embodiment 2
[0181] Example 2. Synthesis of labeled analogs of dimeric PSD-95 inhibitors
[0182] 2.1 Synthesis of fluorophore-labeled analogs (AB143, AB145, AB148, MS23)
[0183] AB145, AB145, AB148, and MS23 to prepare fluorescent ligands. Likewise, coupling of 5-FAM to Ns-deprotected resin-bound AB141 gave AB143. 5-FAM was coupled on a 0.07 mmol scale (NPEG-linker mole) in a total of 2 mL DMF containing N-site-resin / 5-FAM / HATU / collidine in a 1 / 2 / 2 / 3 ratio. For AB145, AB148, or MS23, the coupling time was 6 hours. For AB143, 5-FAM was coupled by continuous coupling for 6 hours and 16 hours, respectively.
[0184] 2.2 15 N, 13 Synthesis of C-labeled dimeric PSD-95 ligand
[0185] use the complete 15 N, 13 Fmoc-protected amino acid synthesis of C-labeled amino acid atoms[ 15 N, 13 C]-PEG4 (IETAV) 2 (AB140) (Cambridge Isotope Laboratories, Inc., Andover, MA, USA). The amino acid building block side chains of Thr and Glu are protected with tert-butyl groups. Labeled Fmoc-Val-OH...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com