Orally disintegrating tablets containing lurasidone and preparation method thereof
A technology of orally disintegrating tablets and lurasidone hydrochloride, which is applied to medical preparations containing active ingredients, pharmaceutical formulas, organic active ingredients, etc., can solve the problems of poor dissolution in vitro and bad taste, and improve compliance , avoid fake medicine, the effect of simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1: Liquidity detection of lurasidone hydrochloride
[0056] According to the above-mentioned fluidity detection method, the fluidity of lurasidone hydrochloride was determined, and the results are as follows:
[0057] The angle of repose of D90≤250um powder is 39.7°;
[0058] The angle of repose of D90≤75um powder is greater than 60°, about 60-80°, the result is poor in repeatability;
[0059] D90≤30um powder cannot pass through the funnel, and the angle of repose cannot be detected.
Embodiment 2
[0060] Example 2 Preparation of orally disintegrating tablets by wet granulation process
[0061] Lurasidone hydrochloride is a white or off-white powder with poor fluidity and a bitter taste, making it difficult to prepare orally disintegrating tablets with good taste. Through a large number of experiments, the inventors were surprised to find that when pH-dependent soluble materials such as acrylic resin E100 and acrylic resin EPO are used as binders, first granulating lurasidone hydrochloride alone or with part of the auxiliary materials can be very good Solve the problem of fluidity and bitterness of this product. When using conventional binder materials, such as povidone k30, hypromellose, ethyl cellulose, 70% ethanol, etc., as the binder of the present invention, although it can solve the problem of poor fluidity, Can not effectively conceal the bitter taste of disintegrating tablets.
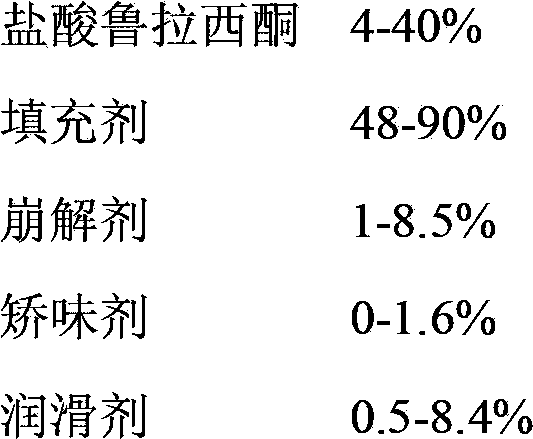
[0062] This experiment uses the prescription: Lurasidone hydrochloride (D90≤75um) 26.7%,...
Embodiment 3
[0068] Example 3 Preparation of orally disintegrating tablets by direct compression of powder
[0069] The powder direct compression method has the advantages of simple process, energy saving and time saving, and is conducive to continuous and automated production. But this process has higher requirements on the fluidity, compressibility and lubricity of the material. In order to meet the requirements of oral disintegration and improved compliance, the material must also have good disintegration or dissolution properties, and a good taste. The challenge is: Lurasidone hydrochloride raw material has poor fluidity and is difficult to dissolve in water. A large amount of fillers must be used to improve it. This may bring about the consequences of large tablets and a large number of tablets. The need to improve the compliance of mental patients with medication is contradictory.
[0070] The inventor investigated the feasibility of direct compression of the powder of lurasidone hydroc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| angle of repose | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com