Application of Anerning granules in preparing medicament for treating viral pneumonia
A technology of viral pneumonia and An'erning granules, applied in the field of medicine, can solve problems such as the research on the treatment of viral pneumonia by An'erning granules, and achieve the effect of protecting immune function and reducing animal death.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 2
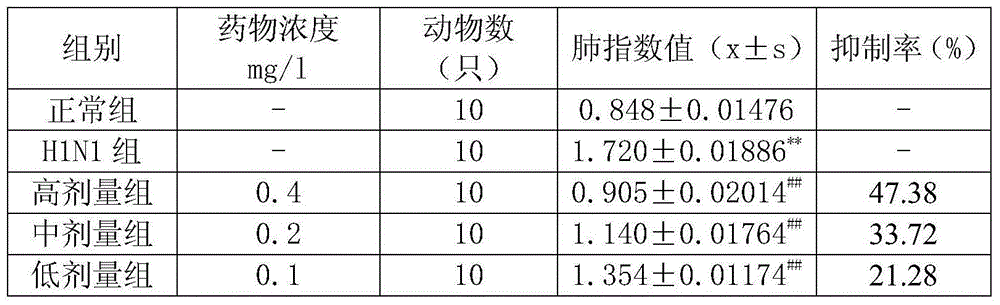
[0047] Experimental example 2, the effect of Anerning Granules on respiratory syncytial virus pneumonia in vivo
[0048] 1. Experimental materials
[0049]Virus strain Respiratory syncytial virus (RSV) Long strain was quoted from the Virus Laboratory of the Institute of Virology, Chinese Academy of Preventive Medicine, and the human laryngeal carcinoma cell line (Hep-2 cells), provided by the Microbiology Laboratory of the Basic Institute of Shandong Academy of Medical Sciences; BALB / c small Rats, 5-7 weeks, half male and half female, body weight 12±1g, SPF grade, cited from Shandong University Animal Center, SPF grade animal license number: SCXX (Lu) 2012004; Ribavirin, Yantai No. 2 Pharmaceutical Factory, batch number 20110514. Anerning Granules were prepared by Jinhe Tibetan Medicine Co., Ltd., batch number 20121119.
[0050] 2. Experimental method
[0051] 1. Virus half infection dose (TCID50)
[0052] Culture with Hep-2 cell monolayer, inoculate 7 concentrations of 10...
Embodiment 1
[0074] Example 1: Anning Granules, 3g per bag, used for the preparation of drugs for the treatment of H1N1 viral pneumonia, 2 bags each time, 3 times a day.
Embodiment 2
[0075] Example 2: Anning Granules, 3g per bag, used for the preparation of drugs for the treatment of H1N1 viral pneumonia, 1 bag each time, 3 times a day.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com