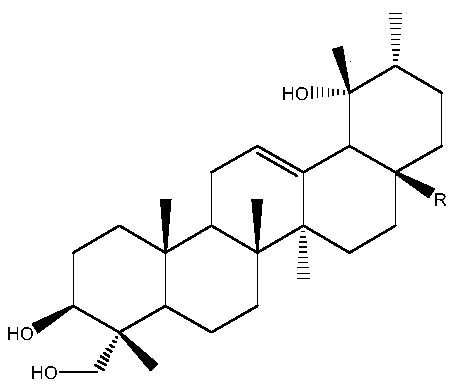
Application of basic acid in the preparation of anti-influenza virus drugs
An influenza virus-rescuing technology of bis-acid, which is applied in the field of bis-rescue to achieve the effect of reducing lung index
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] Example 1 Preparation of the medicinal extract
[0013] Rescue Bing medicinal materials appropriate amount, pulverize, pass through No. 1 sieve, add 8 times the amount of 70% (volume fraction) ethanol to the medicinal materials, extract under reflux for 2 times, 1 hour each time, filter, combine the extracts, put them in a rotary evaporator, and put them in a rotary evaporator. It was concentrated under reduced pressure at 80°C to a thick paste. Transfer the concentrated solution into a drying oven under reduced pressure, continue to dry and pulverize it at 80°C, and obtain the medicinal material extract of Salvation Bing.
Embodiment 2
[0014] Example 2 In vitro influenza virus inhibition experiment
[0015] 1. Experimental materials
[0016] 1.1 Test drug: salvage acid (extracted from salvage, purity > 98%).
[0017] 1.2 Control drugs: ribavirin, oleanolic acid, Jiubing medicinal material extract (see Example 1)
[0018] 1.3 Virus strain: mouse lung-adapted strain of influenza A H1N1 virus (FM / 1 / 47 strain). Chicken embryos were passaged, tested in BSL-3 (Biosafety Laboratory Level 3) laboratory, subpackaged, and stored at -80°C.
[0019] 1.4 Cell Model: Dog Kidney Cell MDCK Cell
[0020] The above medicines were fully dissolved in dimethyl sulfoxide (DMSO) and stored at -20°C for later use.
[0021] 2. Experimental method
[0022] 2.1 Determination of virus virulence
[0023] MDCK cells were pressed 5 x 10 4 96-well culture plates were seeded at a concentration of / mL, 100 μl per well, 37°C, 5% CO 2 Cultivated to form a confluent state of cell monolayers. 100 μl of the 10-fold serial dilution of the...
Embodiment 3
[0032] Example 3 In vivo influenza inhibition experiment
[0033] 1. Experimental materials
[0034] 1.1 Test drug: salvage acid (extracted from salvage, purity > 98%).
[0035] 1.2 Control drugs: Tamiflu, oleanolic acid, and the extract of Jiubing medicinal materials (see Example 1)
[0036] 1.3 Virus: mouse lung-adapted strain of influenza A H1N1 virus (FM / 1 / 47 strain). Chicken embryos were passaged, tested in BSL-3 (Biosafety Laboratory Level 3) laboratory, subpackaged, and stored at -80°C.
[0037] 2. Experimental animals
[0038] 80 BALB / c mice weighing 18g-22g were purchased from Guangdong Provincial Laboratory Animal Center.
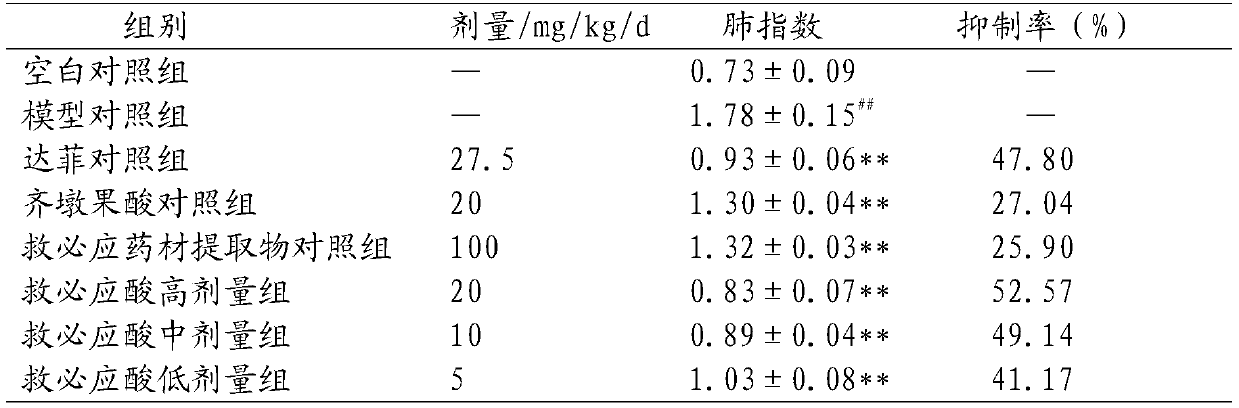
[0039] 3. Experimental method
[0040] BALB / c mice were randomly divided into 8 groups, namely blank control group, model control group, Tamiflu control group, oleanolic acid group, Jiubing medicinal material extract group, and Jiubing acid high, medium and low. 3 dose groups, 10 mice in each group. Except for the blank control group, the a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com