Method and device for preparing vaterite-type calcium carbonate powder
A technology of calcium carbonate powder and vaterite, applied in the direction of calcium carbonate/strontium/barium, etc., can solve the problem of low purity of the vaterite phase, achieve the effects of short cycle, simple raw materials and reaction system, and overcome the low concentration of calcium chloride
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] (1) Add 7.5g of calcium chloride powder into a beaker of 92.5g of distilled water, and after stirring and fully reacting or dissolving, a calcium chloride aqueous solution with a concentration of 7.5% is obtained;
[0034] (2) Add 13.26g of ammonia water into a beaker filled with calcium chloride aqueous solution, stir to make it fully mixed, and obtain a mixed solution with a pH value of 11.2;
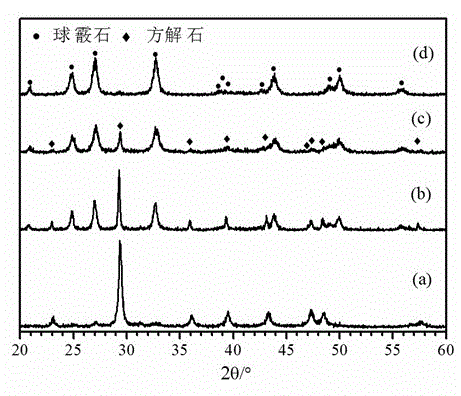
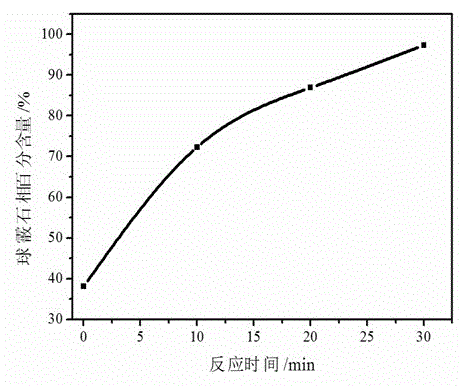
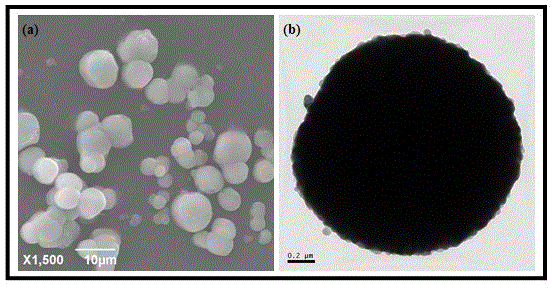
[0035] (3) CO 2The gas is passed into the above-mentioned alkaline solution through the gas micropore diffuser, and calcium carbonate precipitates are gradually formed. Take a sample within 1-2 minutes of just forming the precipitate, and then take a sample every 10 minutes. The obtained samples were tested by XRD, and the results showed that the calcite phase was obtained when the precipitation was just formed, and the vaterite phase was obtained later, and the vaterite phase calcium carbonate powder with negligible content of the calcite phase was obtained after the reaction...
Embodiment 2
[0039] (1) Add 10.5g of calcium chloride powder into a beaker of 89.5g of distilled water, and after stirring and fully reacting or dissolving, a calcium chloride aqueous solution with a concentration of 10.5% is obtained;
[0040] (2) Add 14.08g of ammonia water into a beaker filled with calcium chloride aqueous solution, stir to make it fully mixed, and obtain a mixed solution with a pH value of 11.6;
[0041] (3) CO 2 The gas is passed into the above alkaline solution through the gas micropore diffuser to form calcium carbonate precipitate, and the precipitate is separated from the clear liquid by vacuum filtration device;
[0042] (4) Considering the NH brought in by 14.08g ammonia water 4 + (1.92g, NH in ammonia 4 The concentration of OH is calculated as 26.5%) and Cl - (6.72g) NH produced by the reaction 4 Cl (8.64g) remained in the clear liquid, and in the above clear liquid (theoretical amount of the clear liquid was 111.14g, 108.93g was obtained after filtration,...
Embodiment 3
[0046] (1) Add 7.5g of calcium chloride powder into a beaker of 92.5g of distilled water, and after stirring and fully reacting or dissolving, a calcium chloride aqueous solution with a concentration of 7.5% is obtained;
[0047] (2) Add 13.26g of ammonia water into a beaker filled with calcium chloride aqueous solution, stir to make it fully mixed, and obtain a mixed solution with a pH value of 11.2;
[0048] (3) CO 2 The gas is passed into the above alkaline solution through the gas micropore diffuser to form calcium carbonate precipitate, and the precipitate is separated from the clear liquid by vacuum filtration device;
[0049] (4) Considering the NH brought in by 13.26g ammonia water 4 + (1.81g, NH in ammonia 4 The concentration of OH is calculated as 26.5%) and Cl - (4.8g) NH produced by the reaction 4 Cl (6.61g) remained in the clear liquid, and in the above clear liquid (theoretical amount of the clear liquid was 110.56g, 108.32g was obtained after filtration, co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com