Lithium ion battery anode composite material and its preparation method
A technology for lithium-ion batteries and composite materials, which is applied in the field of lithium-ion battery cathode composite materials containing ferric fluoride and its preparation, and can solve the adverse effects of battery electrode materials on electrochemical performance, difficult removal of surfactants, and easy presence of CTAB, etc. problem, to achieve the effect of obvious charging and discharging platform, good cycle ability and high conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
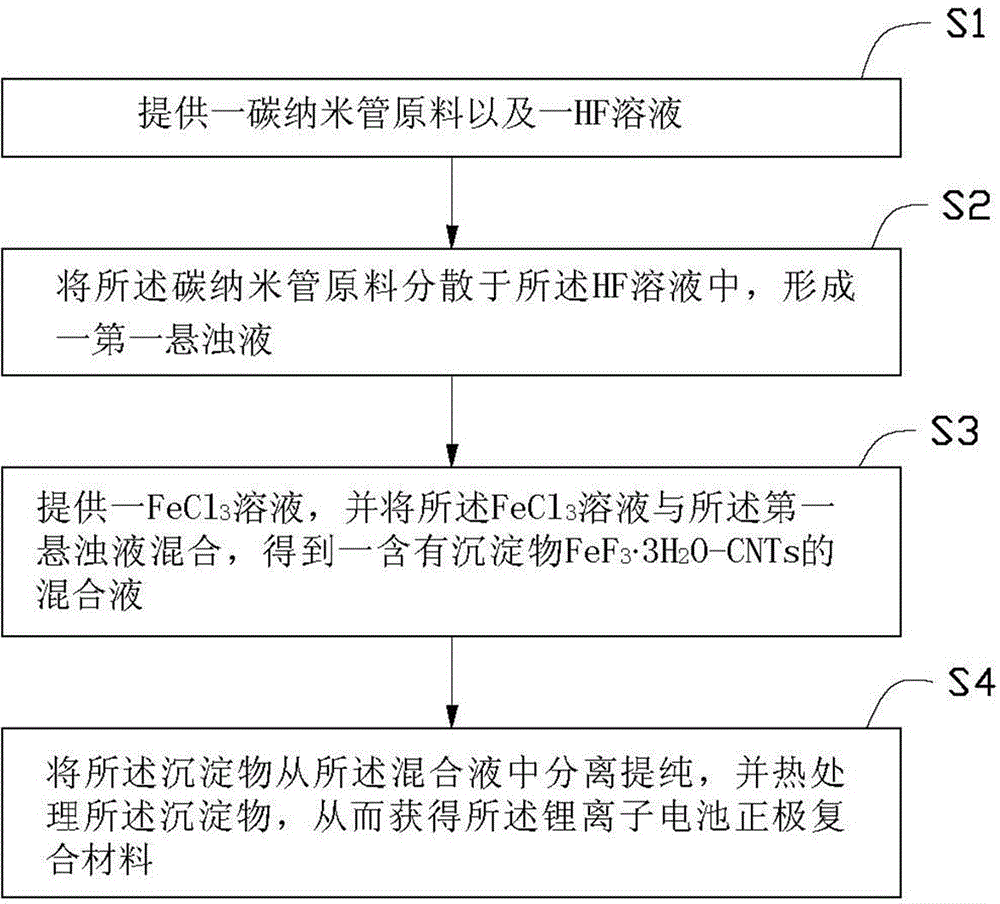
[0020] see figure 1 , the embodiment of the present invention provides a kind of preparation method of lithium-ion battery cathode composite material, it comprises the following steps:
[0021] S1: provide a carbon nanotube raw material and a HF solution;
[0022] S2: Dispersing the carbon nanotube raw material in the HF solution to form a first suspension;
[0023] S3: provide a FeCl 3 solution, and the FeCl 3 The solution is mixed with the first suspension to obtain a precipitate FeF 3 ?3H 2 O-CNTs; and
[0024] S4: Separating and purifying the precipitate, and heat-treating the precipitate, so as to obtain the lithium-ion battery cathode composite material.
[0025] In step S1, the carbon nanotube raw material can be one or more of single-walled carbon nanotubes, double-walled carbon nanotubes or multi-walled carbon nanotubes. The inner diameter of the carbon nanotube is preferably 2 nm to 15 nm, the outer diameter is 10 nm to 30 nm, and the length is about several h...
Embodiment 1
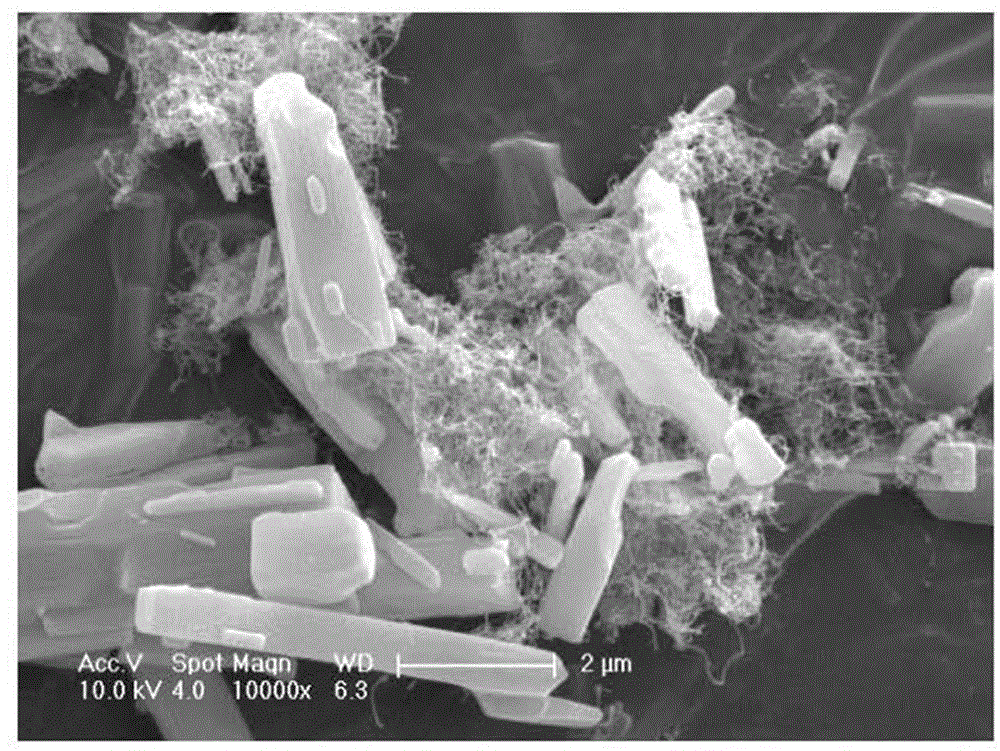
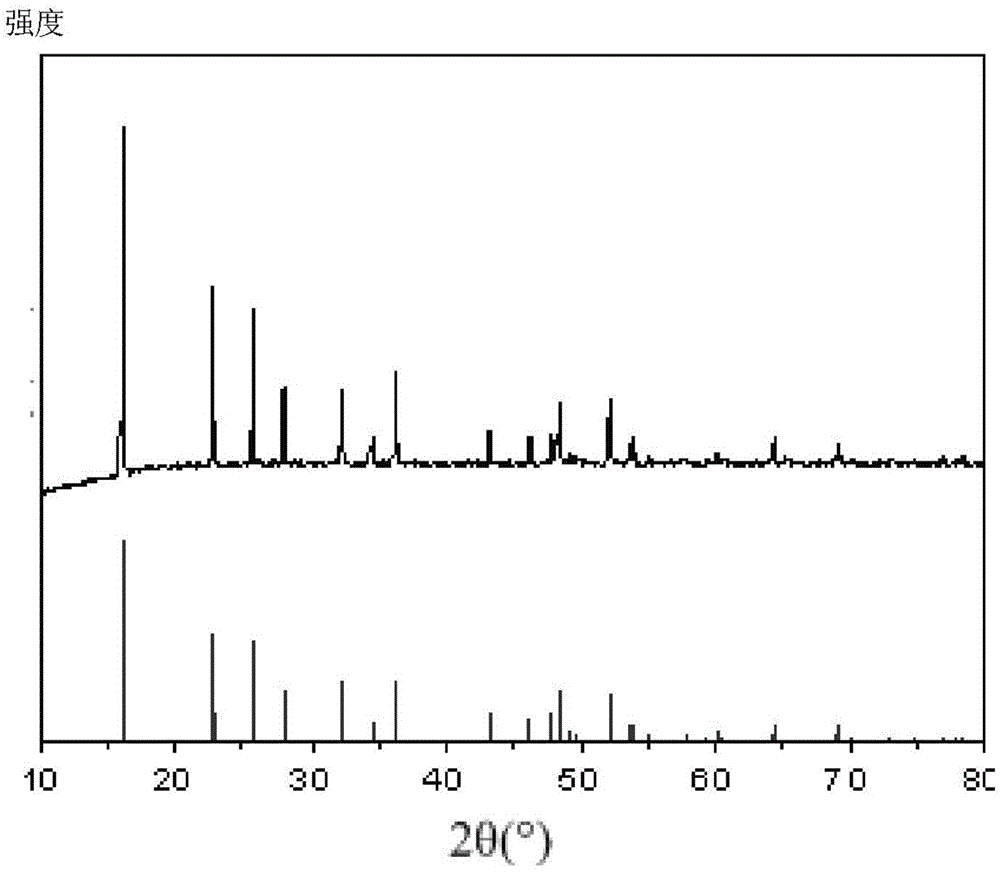
[0032] Add 200 mg of carbon nanotubes into 80 mL of HF (38% by mass) aqueous solution, and ultrasonically disperse for 2 hours to obtain the first suspension; 27.027 g of FeCl 3 .6H 2 O was dissolved in 20 mL deionized water to obtain FeCl 3 solution; under magnetic stirring conditions, the FeCl 3 The solution was added dropwise into the first suspension, and magnetic stirring was continued for 10 h to obtain a mixed solution containing a precipitate; the mixed solution was filtered, and the precipitate was dried at 60° C. for 10 h, and the precipitate was Heat treatment in Ar atmosphere at 125°C for 10h to obtain FeF 3 -CNTs cathode composite material.
Embodiment 2
[0034] Add 300 mg of carbon nanotubes into 40 mL of HF (38% by mass) aqueous solution, and ultrasonically disperse for 1 h to obtain the first suspension; 40.54 g of FeCl 3 .6H 2 O was dissolved in 40 mL deionized water to obtain FeCl 3 solution; under magnetic stirring conditions, the FeCl 3 The solution was added dropwise into the first suspension, and magnetic stirring was continued for 10 h to obtain a mixed solution containing a precipitate; the mixed solution was filtered, and the precipitate was dried at 60° C. for 10 h, and the precipitate was at 130°C in N 2 Heat treatment in the atmosphere for 10h to obtain FeF 3 -CNTs cathode composite material.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com