Porcine contagious pleuropneumonia and streptococcus suis disease combined inactivated vaccine and preparation method thereof
A dual inactivated vaccine and pleuropneumonia technology, applied in the direction of bacteria, antibacterial drugs, bacterial antigen components, etc., can solve problems such as losses and no countermeasures
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
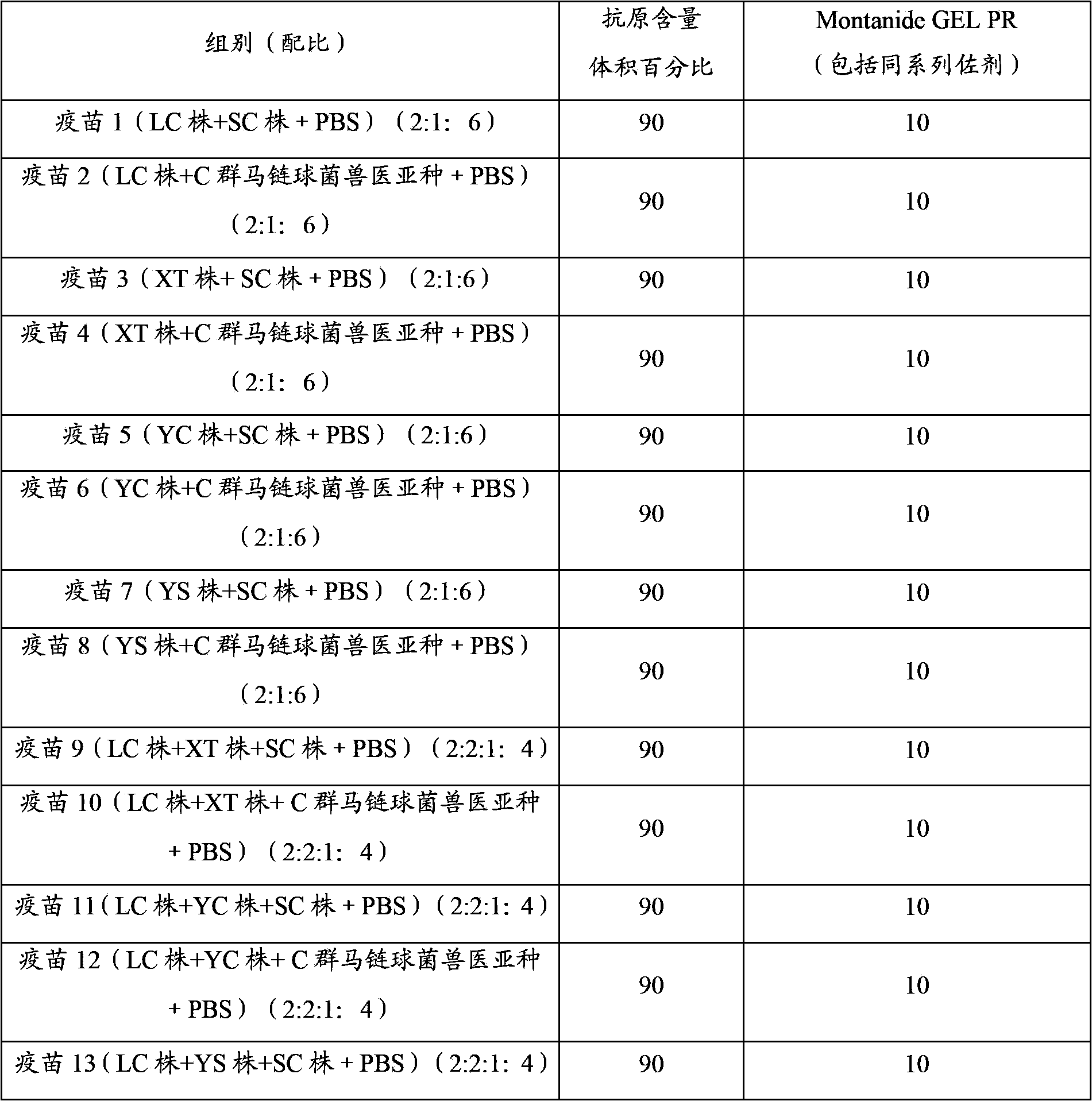
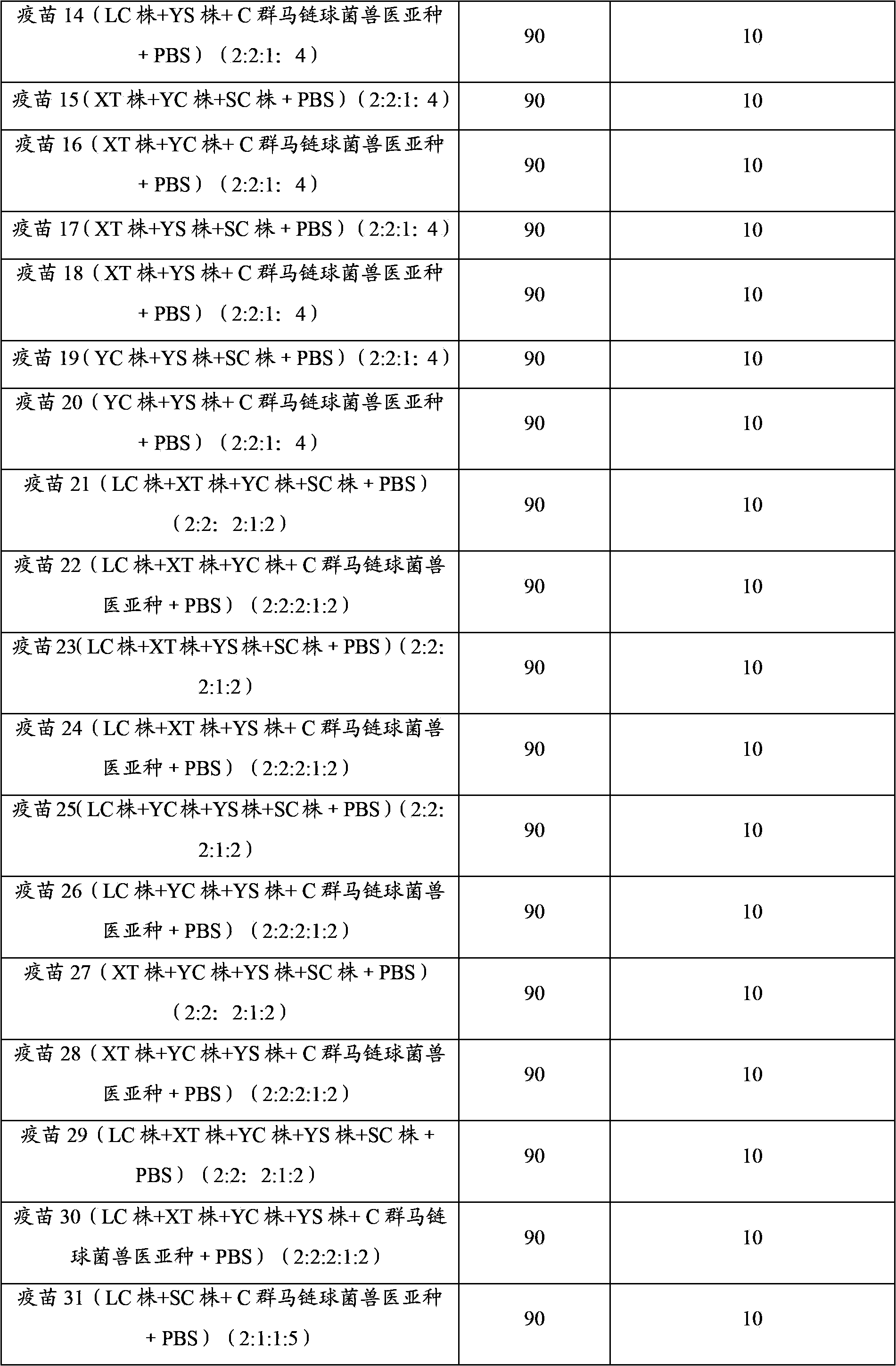
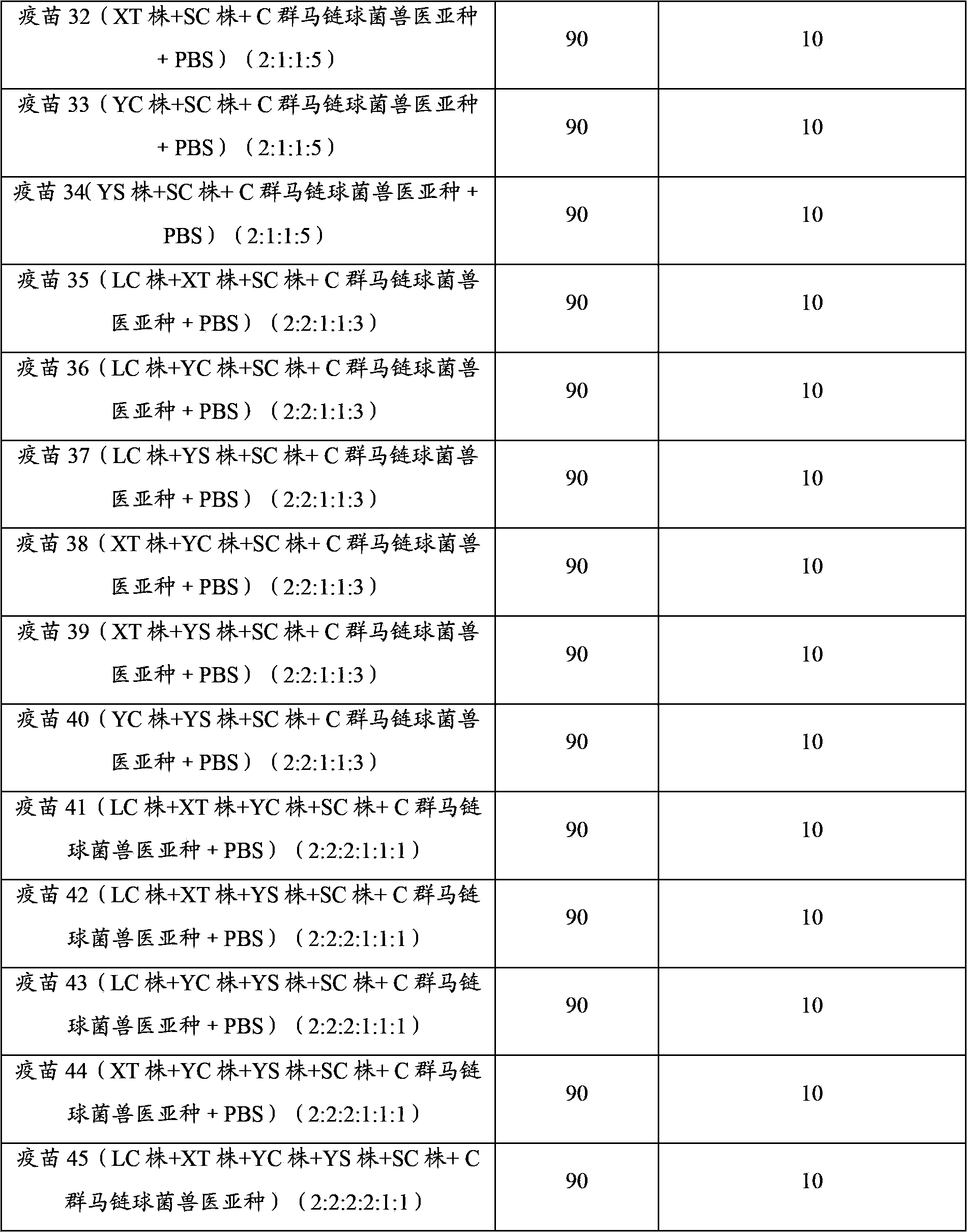
Embodiment 1
[0048] The preparation of embodiment 1 porcine infectious pleuropneumonia, Streptococcus suis dual inactivated vaccine
[0049] 1. Strains
[0050] Actinobacillus pleuropneumoniae serotype 1 LC strain, isolated and identified by Pulaike Bioengineering Co., Ltd., has been preserved in the China Center for Typical Cultures. The preservation date: December 9, 2011, and the preservation number is : CCTCC M 2011458.
[0051] The actinobacillus pleuropneumoniae serotype 2 XT strain is disclosed in Chinese patent CN102391975A, and the preservation number is: CCTCC NO M 2011410.
[0052] Actinobacillus pleuropneumoniae serotype 5 YC strain, isolated and identified by Pulaike Bioengineering Co., Ltd., has been preserved in the China Center for Typical Cultures. The preservation date: December 9, 2011, and the preservation number is : CCTCC M2011459.
[0053] Actinobacillus pleuropneumoniae serotype 7 YS strain, isolated and identified by Pulaike Bioengineering Co., Ltd., has been pr...
Embodiment 2
[0103] Embodiment 2 Porcine infectious pleuropneumonia, streptococcus suis dual inactivated vaccine comparative test
[0104] At present, the inactivated porcine infectious pleuropneumonia vaccines on the market are mainly produced by Wuhan Keqian and China Animal Husbandry Chengdu Pharmaceutical Equipment Factory, and the Streptococcus suis vaccines are mainly produced by Wuhan Keqian, Shanghai Haili, Guangdong Yongshun and Shandong Huahong. . For the vaccines produced by the above companies, we used the same preparation method in this study to prepare several dual inactivated vaccines with the same serotype as the commercially available products, and compared these dual inactivated vaccines. Then, a series of comparisons were made between the optimal dual vaccine obtained after the comparison and the products of the above-mentioned companies sold in the market. Among them, the detailed information of relevant porcine infectious pleuropneumonia vaccines and streptococcal vac...
Embodiment 3 2
[0169] Embodiment 3 dual vaccine and single vaccine combined use comparative test
[0170] Combined with the test results of Example 2, it can be seen that the porcine infectious pleuropneumonia and Streptococcus suis dual inactivated vaccine prepared by this research method can produce effective protection for piglets and pregnant sows. In this example, the optimal combination of porcine infectious pleuropneumonia (serum type 1 LC strain + serum type 5 YC strain + serum type 7 YS strain), Streptococcus suis (Streptococcus equi subspecies zooepidemicus + Streptococcus suis type 2 SC strain) ) dual inactivated vaccine and a commercially available single vaccine were used in combination to conduct an effect comparison test.
[0171] 1. Vaccine preparation and acquisition
[0172] 1.1 Porcine infectious pleuropneumonia (serum type 1 LC strain + serum type 5 YC strain + serum type 7 YS strain), Streptococcus suis (Streptococcus equi subspecies zooepidemicus + Streptococcus suis t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com