Para-nitro aromatic methyl crizotinib hypoxia-activated prodrug for anticancer drugs
A technology of nitroarylmethylcrizotinib and nitrobenzylcrizotinib, applied in the field of p-nitrobenzylcrizotinib, can solve the problem of reduced solubility, poor water solubility, and limited clinical application and other problems, to achieve the effect of high bioavailability, low toxicity and strong tumor selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
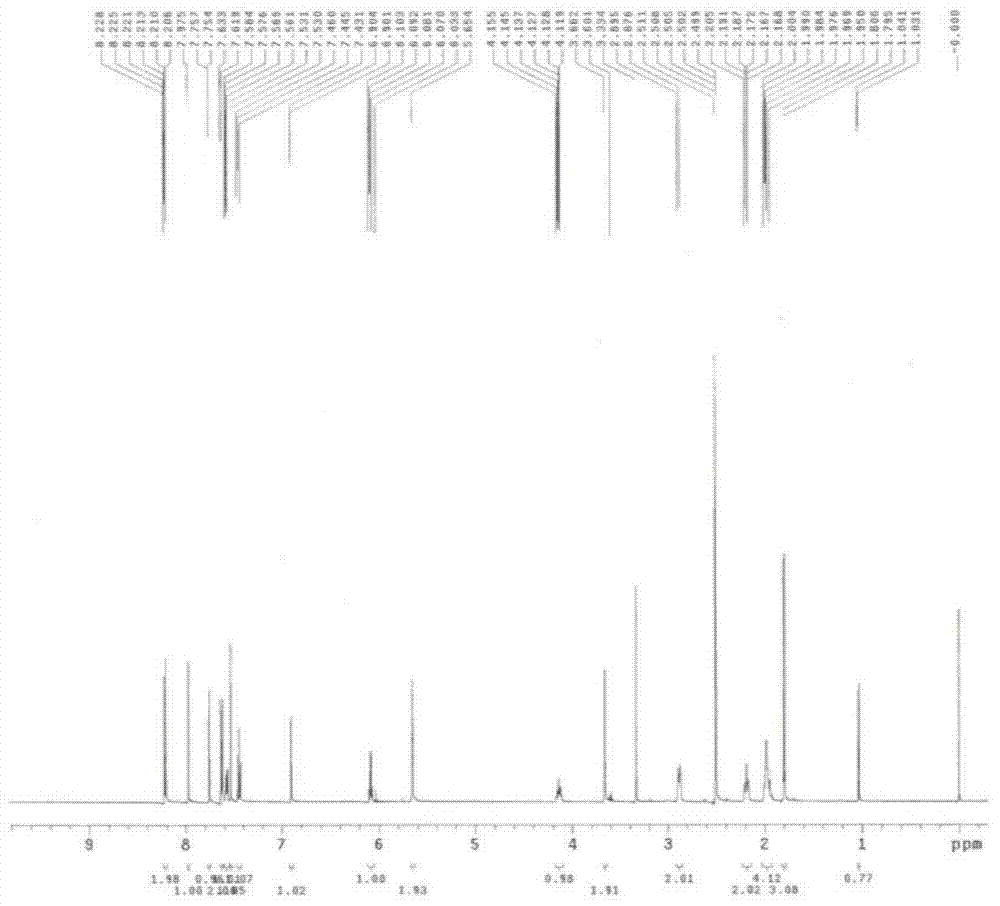
[0105] Embodiment 1. p-nitrobenzyl crizotinib and preparation method thereof
[0106] 1) Chemical name of p-nitrobenzyl crizotinib:
[0107] 5-(1-(1-(4-nitrobenzyl)piperidin-4-yl)-1H-pyrazol-4-yl)-3-((R)-1-(2,6-dichloro -3-fluorophenyl)ethoxy)pyridin-2-amine.
[0108] The chemical structural formula of p-nitrobenzyl crizotinib:
[0109]
[0110] 2) The preferred preparation method of p-nitrobenzyl crizotinib:
[0111] 3.25 g of p-nitrobenzyl bromide and 4.5 g of crizotinib were dissolved in 67.5 ml of dichloromethane, 2.02 g of triethylamine was added dropwise at 25°C, and stirred at 25°C for 3 hours after the addition was complete.
[0112] 75 ml of water, the organic phase was separated, and the aqueous layer was extracted with dichloromethane (50 ml x 3).
[0113] The organic phases were combined and dried over anhydrous sodium sulfate, and the solvent was removed under reduced pressure to obtain a crude product that was recrystallized with dichloromethane and isopro...
Embodiment 2
[0116] Embodiment 2. Comparison of other preparation methods of p-nitrobenzyl crizotinib:
[0117] 4.73 g of p-nitrobenzyl iodide and 4.5 g of crizotinib were dissolved in 90 ml of tetrahydrofuran, and 3.23 g of diisopropylethylamine was added dropwise at 25°C, and stirred at 25°C for 3 hours after the addition was completed.
[0118] 75 ml of water, the organic phase was separated, and the aqueous layer was extracted with dichloromethane (50 ml x 3).
[0119] The combined organic phases were dried with anhydrous sodium sulfate, and the solvent was removed under reduced pressure to obtain a crude product which was recrystallized with dichloromethane and isopropyl ether, and filtered to obtain 3.16 g of a yellow solid, with a yield of 54%.
Embodiment 3
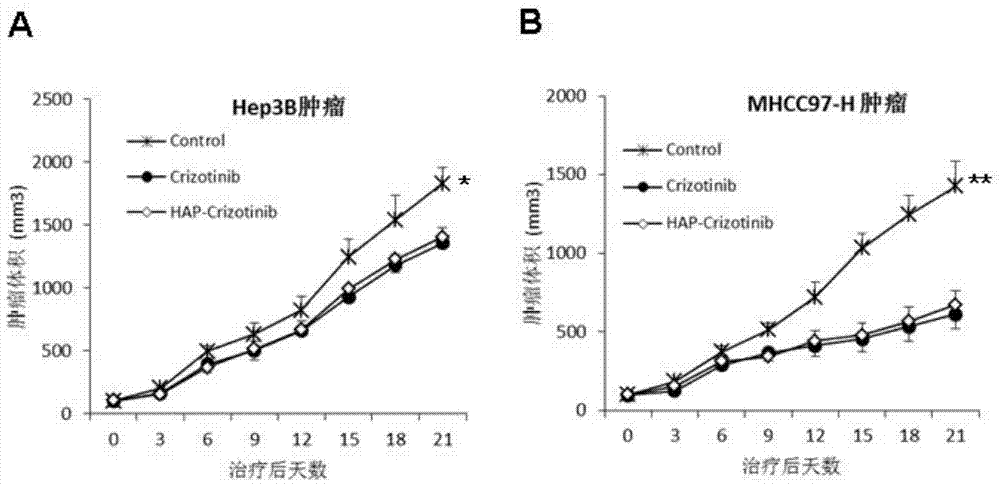
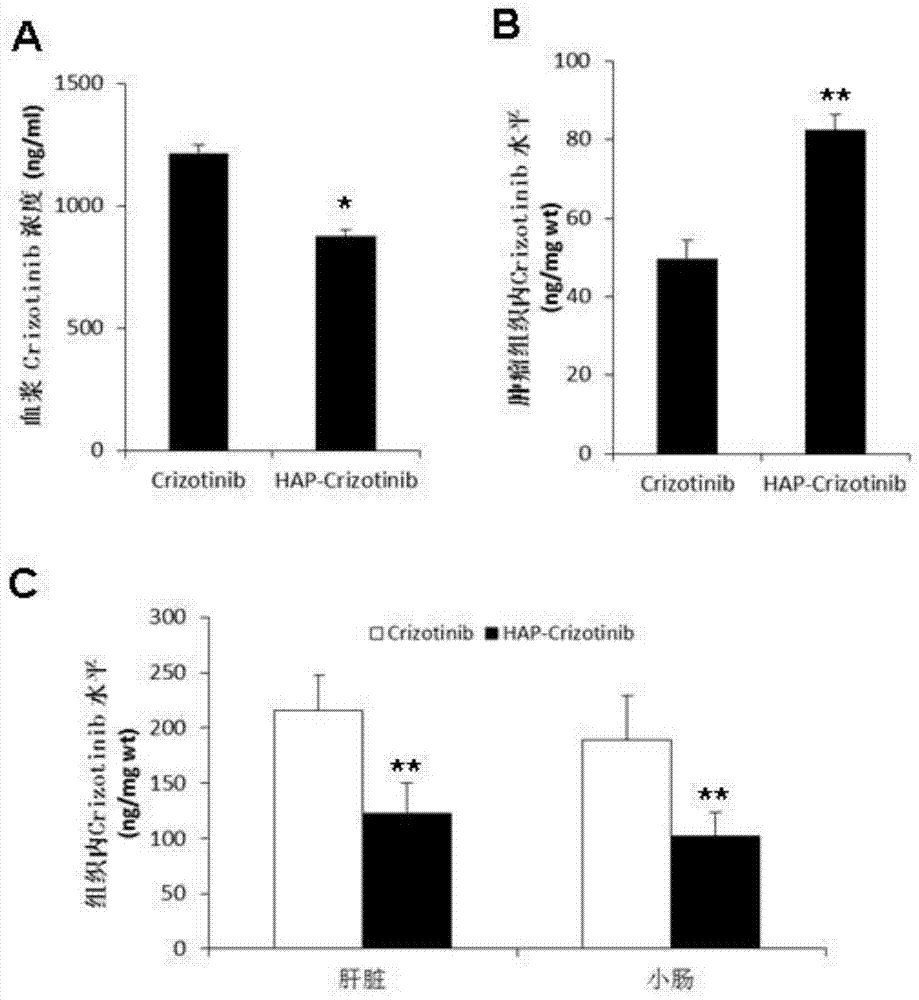
[0120] Example 3. The application effect of nitrobenzyl crizotinib and the comparison with crizotinib
[0121] 1) Identification of anticancer activity of nitrobenzyl crizotinib and comparative analysis of standard drug crizotinib.
[0122] Subcutaneously inject 5×10 6 Human liver cancer cells MHCC97-H and Hep3B cells in the logarithmic growth phase are on the left flank.
[0123] Among them, MHCC97-H cells highly express c-MET, while Hep3B cells express low c-MET (You H, et al.c-Met represents a potential therapeutic target for personalized treatment in hepatocellular carcinoma.Hepatology2011;54:879-9) .
[0124] When the tumor grows to 100mm 3 (day0), the animals were randomly divided into three groups, namely the control group, the crizotinib group and the p-nitrobenzyl crizotinib group, and were given 0.5% hydroxypropyl methylcellulose by intubation Aqueous solution, crizotinib (50 mg / kg) and p-nitrobenzyl crizotinib (50 mg / kg), once daily for 3 weeks.
[0125] Tumor ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com