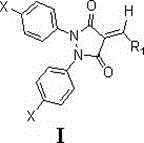
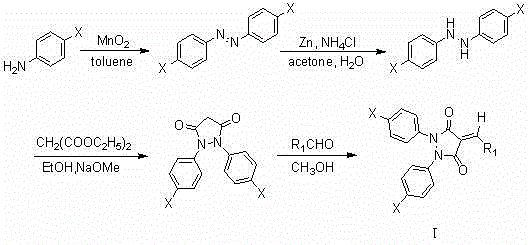
3,5-pyrazoldione derivative containing exocyclic double bond structure unit and preparation method and application thereof
A technology of pyrazole diketone and medicine, which is applied in the field of medicinal chemical synthesis, and achieves the effects of mild reaction conditions, high reaction yield and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Example 1 Preparation of 1,2-di-p-chlorophenyl-4-p-fluorobenzylidene-3,5-pyrazolidinedione
[0016] Add 2.008g (15.74mmol) p-chloroaniline and 50ml toluene into a 100ml three-neck flask, stir to dissolve, install the reflux tube and drying tube, add 6.892g (79.31mmol) MnO 2 , heated to 90°C, kept the temperature for 15 minutes, then raised the temperature to 118°C, and started to reflux. The TLC plate was spotted every 30 minutes to monitor the reaction progress (developing agent petroleum ether: chloroform = 2:1). Stop heating after the reaction is completed, and filter the reaction solution while hot with a sand core funnel covered with silica gel, and wash the filter cake twice with toluene. The filtrate was rotary evaporated to obtain an orange-yellow solid. Continue to wash the filter cake with the toluene evaporated by rotary evaporation, and repeat the operation until the filtrate after washing is colorless. The compound p-chloroazobenzene was obtained.
[00...
Embodiment 2
[0020] Example 2 Preparation of 1,2-di-p-chlorophenyl-4-p-chlorobenzylidene-3,5-pyrazolidinedione
[0021] Substitute p-chlorobenzaldehyde for p-fluorobenzaldehyde, and the preparation method is the same as in Example 1 to obtain a yellow solid compound with a yield of 93.9%, m.p. 204-206°C; 1 H NMR (400 MHz, CDCl 3 ) δ 8.51 (d, J = 8.5 Hz, 2H), 8.11 (s, 1H), 7.51 (d, J = 8.5 Hz, 2H), 7.40 (dd, J = 9.1, 2.2 Hz, 2H), 7.37 (dd, J = 9.1, 2.2 Hz, 2H), 7.31 (d, J = 8.9 Hz, 2H), 7.31(d, J = 8.9 Hz, 2H) . 13 C NMR (101 MHz, CDCl 3 ) Δ 163.51, 161.84, 153.06, 141.23, 136.22, 134.87, 134.77, 132.47, 132.20, 130.73, 129.46, 129.24, 123.74, 116.89. HRMS (ESI): m / z cack. 22 h 13 Cl 3 N 2 o 2 (M+H) + , 443.0121,found, 443.0126.
Embodiment 3
[0022] Example 3 Preparation of 1,2-di-p-chlorophenyl-4-p-bromobenzylidene-3,5-pyrazolidinedione
[0023]P-bromobenzaldehyde was substituted for p-fluorobenzaldehyde, and the preparation method was the same as in Example 1 to obtain a yellow solid compound with a yield of 94.2%, m.p. 191-192°C; 1 H NMR (400 MHz, CDCl 3 ) δ 8.39 (d, J = 8.6 Hz, 2H), 8.07 (s, 1H), 7.66 (d, J = 8.6 Hz, 2H), 7.35 (dd, J = 9.1, 2.2 Hz, 2H), 7.35 (dd, J = 9.1, 2.2 Hz, 2H), 7.30 (d, J = 8.9 Hz, 2H), 7.30(d, J = 8.9 Hz, 2H). 13 C NMR (101 MHz, CDCl 3 ) Δ 161.41, 160.65, 152.11, 140.32, 135.13, 133.76, 133.24, 131.34, 131.11, 129.66, 128.44, 128.16, 122.65, 122.23. HRMS (ESI): m / z CACLD. for C 22 h 13 Cl 2 N 2 o 2 Br (M+H) + , 486.9616,found, 486.9612.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com