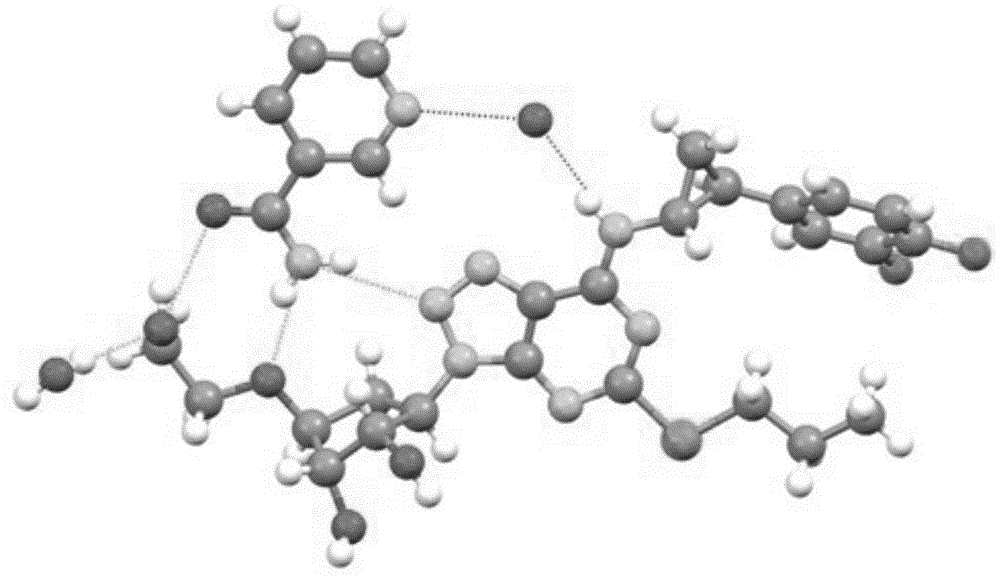
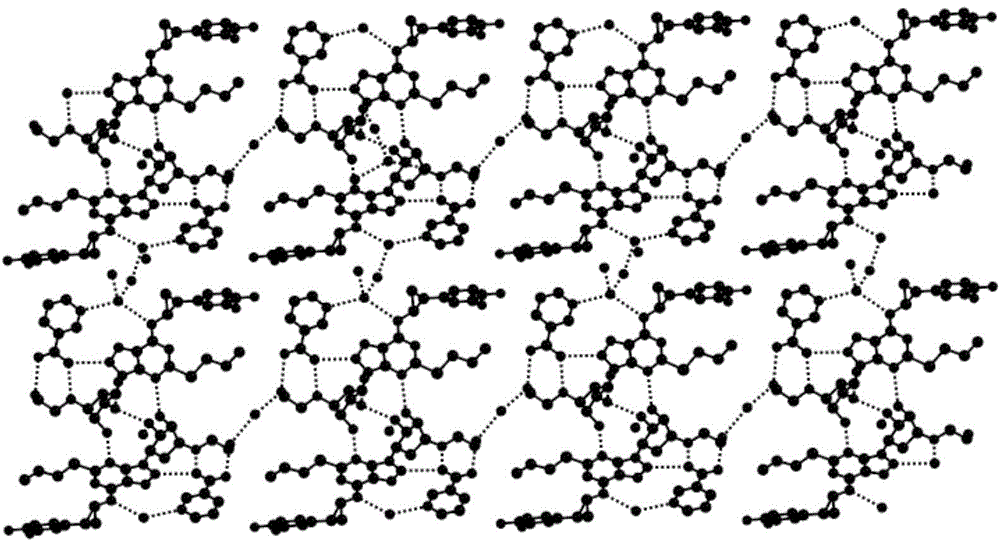
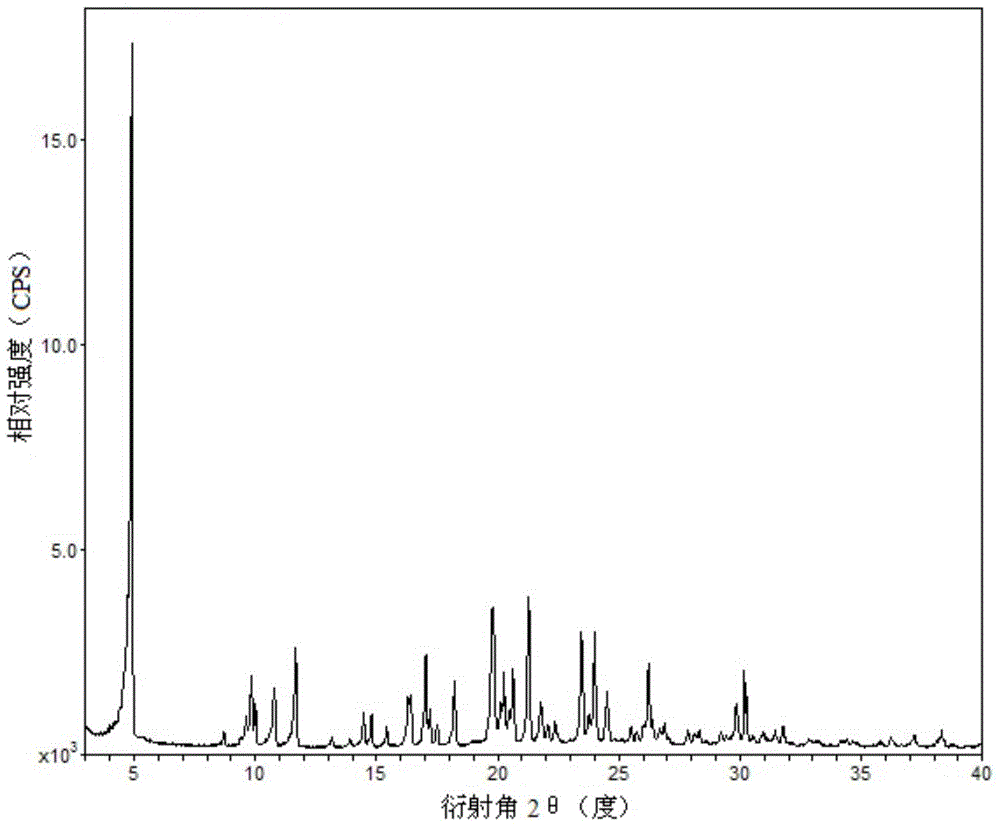
Ticagrelor pharmaceutical cocrystals and preparation methods thereof
A technology of ticagrelor and drugs, applied in the field of preparation of new ticagrelor organic drug co-crystals and drug co-crystals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Example 1: Add 0.3g of ticagrelor and 0.07g of nicotinamide into a solution of 5ml of ethyl acetate, heat to reflux at 75°C, stir to dissolve, then cool down naturally, and slowly precipitate white crystals after reaching room temperature, 48 After 1 hour, it was filtered to obtain the white organic drug cocrystal 1 of ticagrelor.
Embodiment 2
[0070] Example 2: Add 0.6g and 0.14g of ticagrelor and nicotinamide to 15ml of ethyl acetate solution, heat to 40°C, stir and dissolve, then place at room temperature, slowly crystallize, and obtain white Organic pharmaceutical cocrystal of ticagrelor 1.
Embodiment 3
[0071] Example 3: Dissolve 1.5g of ticagrelor and 0.21g of nicotinamide in a mixed solvent of 10ml of acetone and ethyl acetate (volume ratio of acetone and ethyl acetate (mL / mL=1:4)), and heat to Reflux at 50°C, stir to dissolve, and when the solution is clear, stop heating, cool down naturally, and slowly crystallize to obtain ticagrelor organic drug co-crystal 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com