Transparent medical thermoplastic rubber plug and preparation method thereof
A thermoplastic and transparent technology, applied in the field of medical infusion packaging, can solve the problems of difficult to find black spots and foreign bodies, many processes, long cycle, etc., achieve excellent stability and biocompatibility, and improve the performance of puncture and chip removal.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
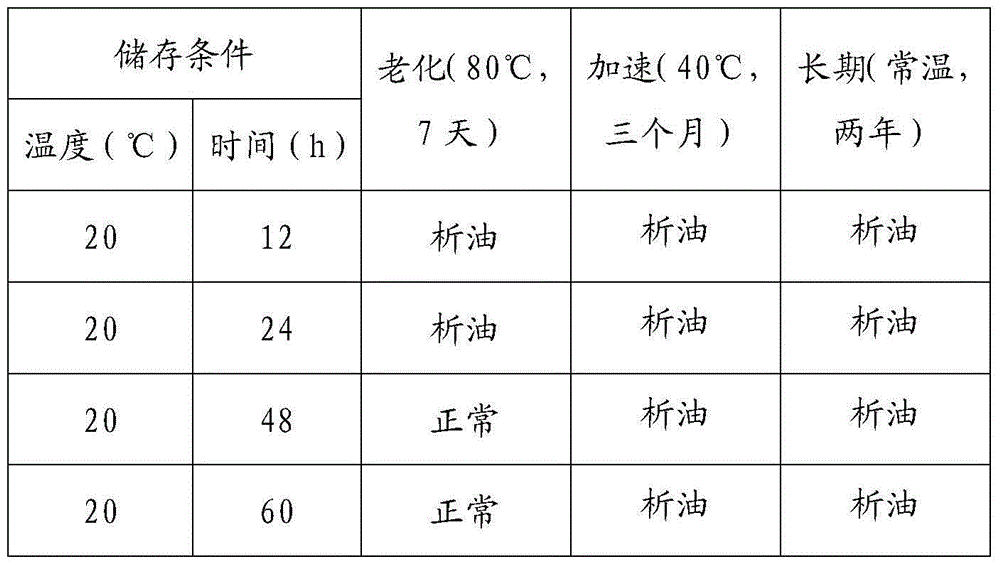
[0040] In the preparation method adopted in the present invention, before the raw materials are extruded and granulated, the high-speed dry-blended mixture is pre-stored at 30-40°C for 24-48 hours to ensure the infiltration compatibility and mutual compatibility between the mineral oil and the resin. The formation of the transmission network structure, thereby improving the stability of the rubber plug. Now compare some experimental data as follows:
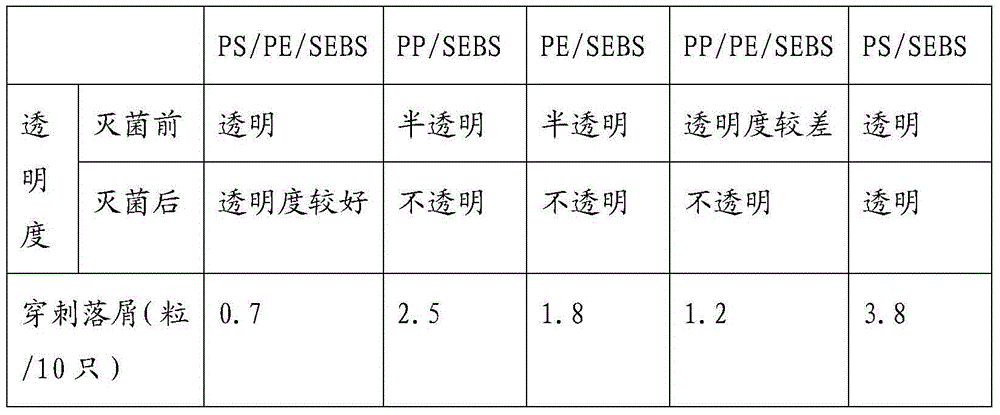
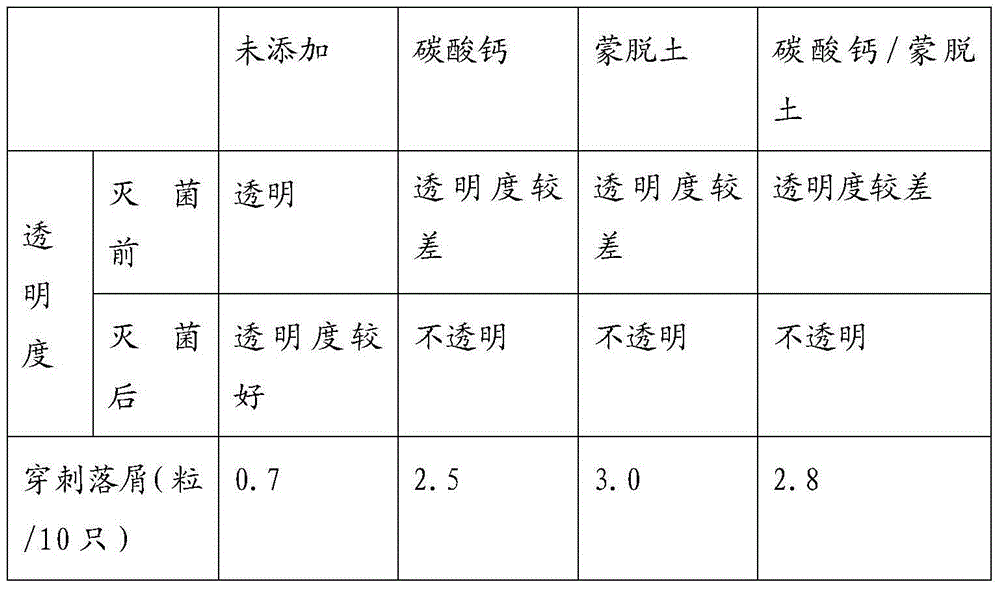
[0041] Table 3: Comparison Table of Stability Performance Test Results
[0042]
[0043]
[0044] The mixed storage results of raw materials in Table 3 and the results of rubber stopper stability investigation show that the best pre-storage conditions are at a temperature of 30°C-40°C and a time of 24-48h.
[0045] The invention has a simple process for preparing the medical thermoplastic rubber stopper, the cost is similar to that of the isoprene rubber stopper and the butyl rubber stopper, and has great market prospect. ...
Embodiment 1
[0049] A medical thermoplastic rubber stopper material, mainly made of the following raw materials in parts by weight: 5 parts of polystyrene, 15 parts of polyethylene, 55 parts of SEBS, 24.8 parts of mineral oil, tetrakis[3-(3,5-di-tert-butyl 0.1 part of pentaerythritol ester (Irganox 1010) and 0.1 part of tris(2,4-di-tert-butylphenyl) phosphite (Irgafox 168).
[0050] The preparation method comprises the following steps:
[0051] a: First blow dry polystyrene, polyethylene, and SEBS at 40°C for 3 hours;
[0052] b: Weigh dry polystyrene, polyethylene, SEBS and mineral oil, antioxidant;
[0053]c: dry-mix the raw materials in step b at high speed to obtain a mixture, and pre-store at 30°C for 48 hours;
[0054] d: Add the mixture in step c to a twin-screw extruder, melt blend, and granulate, wherein the temperature of the first zone of the twin-screw extruder barrel is 180°C, the temperature of the second zone is 180°C, and the temperature of the third zone is 185°C. The t...
Embodiment 2
[0057] A medical thermoplastic rubber stopper material, mainly made of the following raw materials in parts by weight: 15 parts of polystyrene, 5 parts of polyethylene, 55 parts of SEBS, 24.6 parts of mineral oil, tetrakis[3-(3,5-di-tert-butyl 0.2 parts of pentaerythritol ester (Irganox 1010) and 0.2 parts of tris(2,4-di-tert-butylphenyl) phosphite (Irgafox 168).
[0058] The preparation method is the same as in Example 1, and will not be repeated here.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com