Preparation method of p-aminophenyl-beta-ethoxyl sulphone sulphate
A technology of p-aminophenyl and hydroxyethyl sulfone, applied in the field of preparation of p-aminophenyl-β-hydroxyethyl sulfone sulfate, to achieve the effects of reducing the discharge of three wastes, being conducive to environmental protection, and reducing the amount of use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
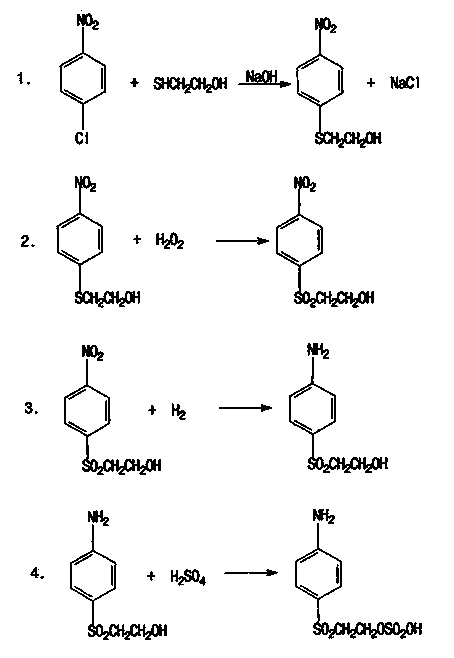
[0036] a. First add N,N-dimethylformamide solvent into the reaction kettle, then add p-nitrochlorobenzene and mercaptoethanol to fully dissolve and mix well, then slowly add sodium hydroxide to control the amount of sodium hydroxide After 4 hours of addition, the molar ratio of p-nitrochlorobenzene, mercaptoethanol, N,N-dimethylformamide and sodium hydroxide is 1:1.2:1.5:1; Control the temperature at 70±2°C, react for 4.0 hours under this temperature condition, and obtain the reaction product after the reaction is completed; filter the obtained reaction product through a filter pool at a temperature of <40°C, remove the solid NaCl, and obtain the filtrate. The filtrate was distilled under reduced pressure at 80-120mmHg and 130-140°C for 1.5h to distill off water and N,N-dimethylformamide to obtain p-nitrophenyl-β-hydroxyethyl sulfide;
[0037] b. Add the p-nitrophenyl-β-hydroxyethyl sulfide and sodium tungstate catalyst obtained in step a into the oxidation kettle, and slowly ...
Embodiment 2
[0043] In step a: control the addition of sodium hydroxide for 3.5 hours, and the molar ratio of the addition amount of p-nitrochlorobenzene, mercaptoethanol, N,N-dimethylformamide and sodium hydroxide is 1:1.1:1.6 : 1; after the addition of sodium hydroxide, the temperature is controlled to be 75±2°C, and the reaction is carried out at this temperature for 3.5h;
[0044] In step b: the molar ratio between hydrogen peroxide and p-nitrophenyl-β-hydroxyethyl sulfide is 1.05:1, and the addition of sodium tungstate catalyst accounts for 0.3% of the mass of sulfide; during the process of adding hydrogen peroxide, the temperature is controlled at 80-85°C, and the continuous addition time of hydrogen peroxide is 4h; after adding hydrogen peroxide, react at 95-100°C for 2h;
[0045] In step c: repeatedly washing the oxidized crystal material obtained in step b with methanol having a concentration of 20-30% by mass;
[0046] In step d: the mass ratio between the oxidized crystallizati...
Embodiment 3
[0049] In step a: control the addition of sodium hydroxide for 3 hours, the molar ratio of the addition amount of p-nitrochlorobenzene, mercaptoethanol, N,N-dimethylformamide and sodium hydroxide is 1:1.15:2.0: 1. After adding sodium hydroxide, control the temperature to 78-80°C, and react at this temperature for 3 hours; carry out vacuum distillation of the obtained filtrate at 120-150mmHg, 120-130°C for 1.5h;
[0050] In step b: the molar ratio between hydrogen peroxide and p-nitrophenyl-β-hydroxyethyl sulfide is 1.1:1, and the amount of sodium tungstate catalyst added accounts for 0.1% of the mass of sulfide; during the process of adding hydrogen peroxide, the temperature is controlled at 95±2°C, and the continuous addition time of hydrogen peroxide is 5h; after adding hydrogen peroxide, react at 95-100°C for 3h;
[0051] In step c: repeatedly washing the oxidized crystal material obtained in step b with methanol having a concentration of 60-70% by mass;
[0052] In step d...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap