Identification method for Tibetan herb Saibei Corydalis
An identification method and a technology for the medicinal material of Corydalis are applied in the field of identification of the Tibetan medicinal material Corydalis saibei, which can solve the problems of backward quality standards of Corydalis saibei, inability to accurately and effectively identify Corydalis saibei, lack of content determination indicators, etc. , The identification method is simple and the effect of ensuring the accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
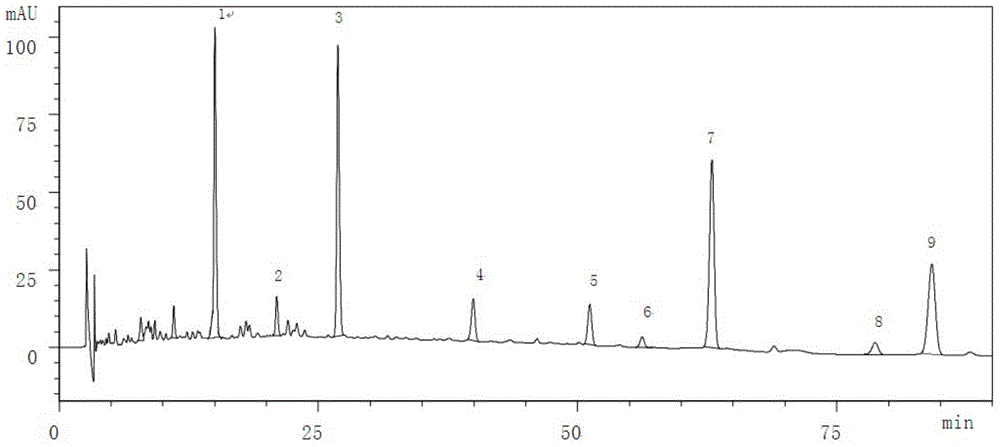
[0084] The Tibetan medicinal material Corydalis Saibei was provided by Qinghai Jinhe Tibetan Medicine Pharmaceutical Co., Ltd.
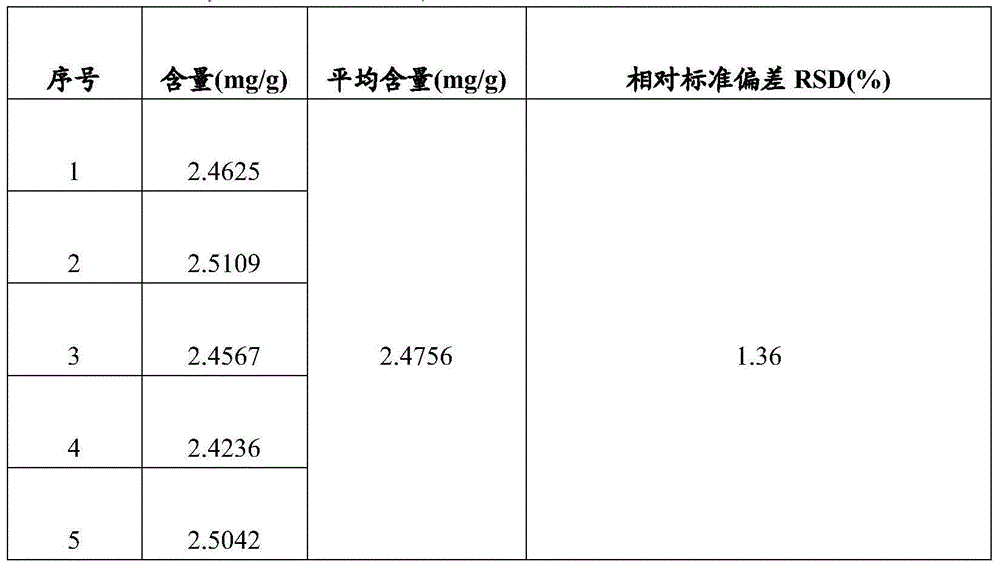
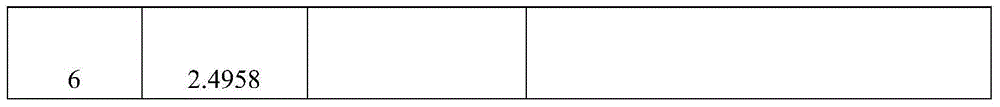
[0085] Determination of the content of total alkaloids:
[0086] Take about 10g of the fine powder of this product, accurately weigh it, put it into a stoppered conical flask, add 50ml of chloroform-ammonia water mixed solution with a volume ratio of 100:1, weigh it, ultrasonic for 30min, and place it overnight (12 hours) , filtered, the residue was added to 50ml of chloroform-ammonia mixed solution with a volume ratio of 100:1, shaken for 1 hour, filtered, and the residue was washed 3 times with a chloroform-ammonia mixed solution with a volume ratio of 100:1, each time 15m, filter; combine the above filtrate and lotion, evaporate to dryness at low temperature and reduced pressure (below 40°C), add 10ml of ethanol to the residue to dissolve, add 1g of activated carbon, shake for 10min to decolorize, filter, and accurately measure 5ml of subsequent f...
Embodiment 2
[0099] The Tibetan medicinal material Corydalis Saibei was provided by Qinghai Jinhe Tibetan Medicine Pharmaceutical Co., Ltd.
[0100] Determination of the content of total alkaloids:
[0101] Take about 8g of the medicinal powder of this product, accurately weigh it, put it in a stoppered Erlenmeyer flask, add 50ml of chloroform-ammonia water mixed solution with a volume ratio of 100:1, weigh it, ultrasonic for 30min, let stand overnight, filter, Add 40ml of chloroform-ammonia water mixed solution with volume ratio of 100:1 to the residue, shake for 1 hour, filter, wash residue with 100:1 volume of chloroform-ammonia water mixed solution for 3 times, each time 15m, filter Combine the above filtrate and lotion, evaporate to dryness at low temperature and reduced pressure (below 40°C), add 10ml of ethanol to the residue to dissolve, add 1g of activated carbon, shake for 10min to decolorize, filter, and accurately measure 5ml of the filtrate to the Erlenmeyer flask. Accurately...
Embodiment 3
[0114] The Tibetan medicinal material Corydalis Saibei was provided by Qinghai Jinhe Tibetan Medicine Pharmaceutical Co., Ltd.
[0115] Determination of the content of total alkaloids:
[0116] Take about 12g of the medicinal material fine powder of this product, accurately weigh it, put it in a stoppered conical flask, add 50ml of chloroform-ammonia water mixed solution with a volume ratio of 100:1, weigh it, ultrasonic for 30min, let stand overnight, filter, Add 60ml of chloroform-ammonia water mixed solution with a ratio of 100:1 by volume to the residue, shake for 1 hour, filter, wash the residue with 100:1 chloroform-ammonia mixed solution for 3 times, each time 15m, filter Combine the above filtrate and lotion, evaporate to dryness at low temperature and reduced pressure (below 40°C), add 10ml of ethanol to the residue to dissolve, add 1g of activated carbon, shake for 10min to decolorize, filter, and accurately measure 5ml of the filtrate to the Erlenmeyer flask. Accur...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com