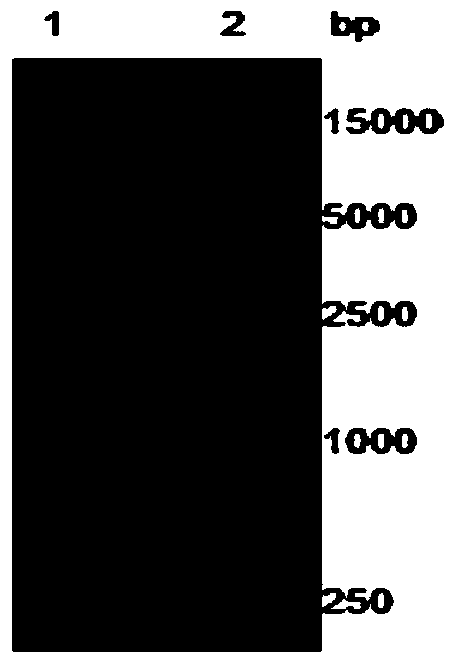Respiratory syncytial virus-like particles and preparation method and application thereof
A technology for syncytial virus and respiratory tract, which is applied to respiratory syncytial virus-like particles and the fields of preparation and application thereof, can solve problems such as low expression level, allergic reaction, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Embodiment 1: Construction of recombinant shuttle plasmid
[0051] 1. Construction: In view of the codon preference of mammalian cells, optimize the codons of wild-type RSV M, F, and G, and name them Msyn, Fsyn, and Gsyn, respectively, to increase their expression in mammalian cells; Whole-gene synthesis of codon-optimized M, F, and G genes, pMA-T-Msyn, pMA-T-Fsyn, and pMA-T-Gsyn; use restriction endonucleases HindⅢ and EcoRⅤ to cut pMA-T-Msyn respectively , pMA-T-Fsyn and pMA-T-Gsyn, and recover the target fragments.
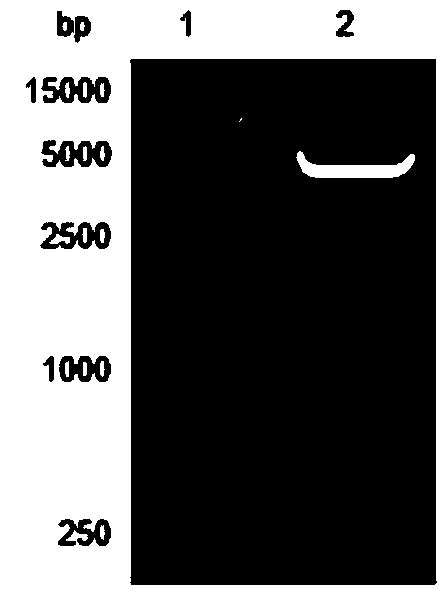
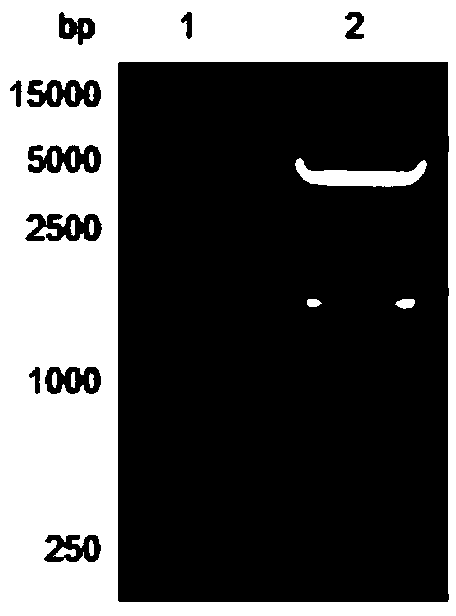
[0052] After agarose gel electrophoresis, near 774bp (see figure 1 ) and 1728bp (see figure 2 ) each has a specific band, which is consistent with the length of Msyn gene and Fsyn gene respectively.
[0053] The shuttle vector pShuttle-CMV was cut with restriction endonucleases HindⅢ and EcoRⅤ, the vector fragments were recovered, and the target fragments Msyn, Fsyn, and Gsyn were ligated overnight at 12°C to 16°C, and the ligation products were name...
Embodiment 2
[0073] Embodiment 2: Construction contains the recombinant adenovirus plasmid of Msyn, Fsyn or Gsyn gene
[0074] 1. In RecA + Homologous recombination in E.coli BJ5183 cells:
[0075] Linearize pShuttle-Msyn, pShuttle-Fsyn, pShuttle-Gsyn with PmeⅠ enzyme digestion, digest overnight at 37°C. Take about 500ng of pShuttle-Msyn DNA molecules, pShuttle-Fsyn DNA molecules, pShuttle-Gsyn DNA molecules after linearization with PmeⅠ, add H 2Add O to 400 μl, add 40 μl 3mol / L sodium acetate (pH 5.2), then add 2-2.5 times the volume of absolute ethanol, and place at -20°C for 30 minutes to precipitate. Centrifuge at 12000rpm for 10min at 4°C. The pellet was washed once with 75% ethanol, dried and dissolved in 10 μl ddH 2 O and stored at -20°C for later use. Take about 5 μl of the pShuttle-Msyn DNA molecules, pShuttle-Fsyn DNA molecules, and pShuttle-Gsyn DNA molecules obtained in the previous step, and chemically transform the RecA containing the adenovirus backbone plasmid pAdEasy-...
Embodiment 3
[0090] Example 3: Transfection of recombinant adenovirus plasmid into 293 cells, cell culture, and obtaining recombinant adenovirus
[0091] 1. Digest recombinant adenoviral plasmids pAdEasy-Fsyn, pAdEasy-Msyn and pAdEasy-Gsyn with Pac I:
[0092] Recombinant adenoviral plasmids pAdEasy-Fsyn, pAdEasy-Msyn, pAdEasy-Gsyn were digested overnight with Pac I, digested completely to remove plasmid components such as ori and Kana resistance genes, and exposed their ITRs, after digestion, precipitated with absolute ethanol, dried, 20 μl sterile HO 2 O dissolved.
[0093] 2. Transfection of 293 cells
[0094] 293 cells were donated by the Institute of Viral Disease Prevention and Control, Chinese Center for Disease Control and Prevention.
[0095] When the growth abundance of 293 cells is about 50% to 70%, take 10 μl each of the recombined adenovirus plasmids pAdEasy-Fsyn, pAdEasy-Msyn and pAdEasy-Gsyn digested with Pac I enzyme and mix well with 240 μl opti-MEM (GIBCO) ( Solution ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com