Fermentation production method of rifamycin SV based on oxygen uptake rate OUR used as control parameter
A technology of rifamycin and oxygen consumption rate, applied in the field of fermentation production of rifamycin SV, can solve problems such as no technical guidance scheme is given, and achieve the effects of improving fermentation production level, improving conversion rate and promoting synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1: The fermentative production method of rifamycin SV of the present invention is to use Nocardia mediterranei as a starting strain, and generate rifamycin SV through purebred culture and three-stage fermentation. The specific process steps include the preparation of inclined planes process, mother bottle seed preparation process, first-level seed preparation process, second-level seed preparation process, and fermentation preparation process. Except for the fermentation preparation process, other processes are prior art. The further processing steps of each operation of the present invention are as follows:
[0046] (1) The preparation process of the slope: ①Preparation of the mother slope: according to the requirements of aseptic operation, pick an appropriate amount of sandy soil seeds with an inoculation needle, and evenly spread it on the slope medium. It is required to be uniform, sparse, and easy to select. Cultivate at a constant temperature for 9-10...
Embodiment 2
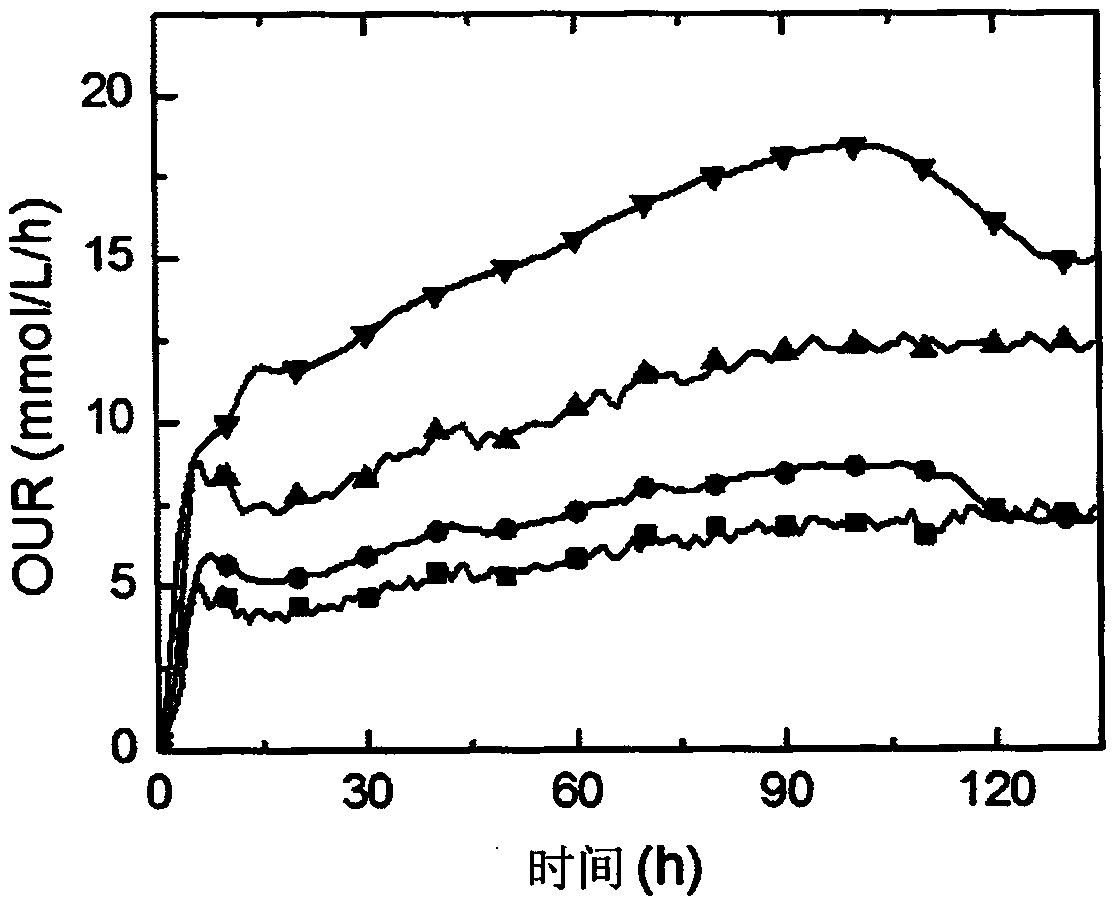
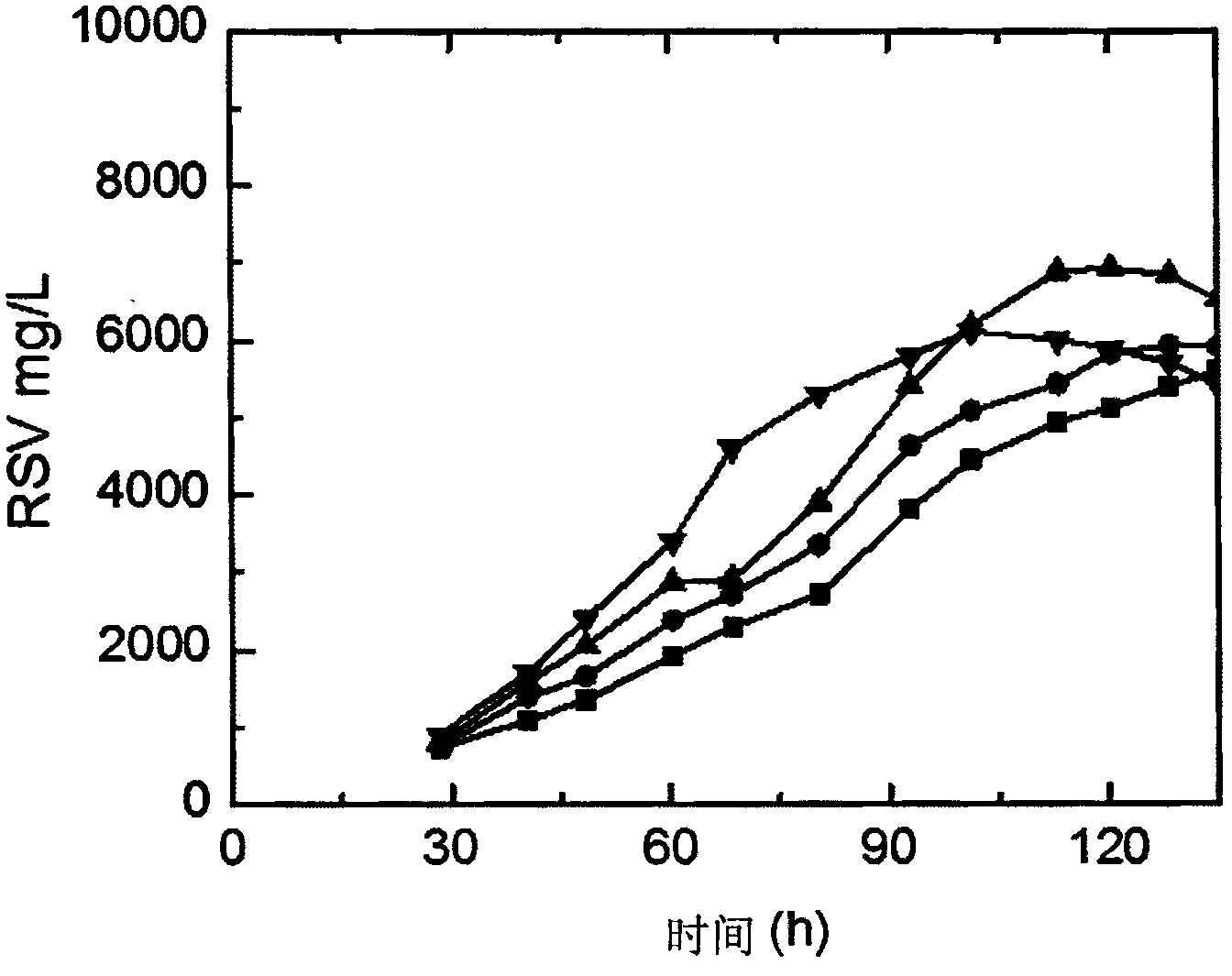
[0049] Embodiment 2: the fermentative production method of rifamycin SV of the present invention is to use Nocardia mediterranei as a starting strain, and generate rifamycin SV through purebred culture and three-stage fermentation, and the specific process steps include inclined plane Preparation process, mother bottle seed preparation process, primary seed preparation process, secondary seed preparation process, fermentation preparation process. In the fermentation preparation process, different oxygen supply levels are achieved by controlling the stirring speed and ventilation flow rate of the fermenter during the fermentation process, specifically within 74 to 75 hours in the early stage of the fermentation process (stirring speed is 550rpm, and the ventilation ratio of the fermenter is 1 : 0.8~0.9vvm) to maintain a high level of oxygen consumption rate OUR in the fermentation system is 20mmol / L / h (or 18 mmol / L / h), so that the titer rises rapidly, after 74~75 hours in the ea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com