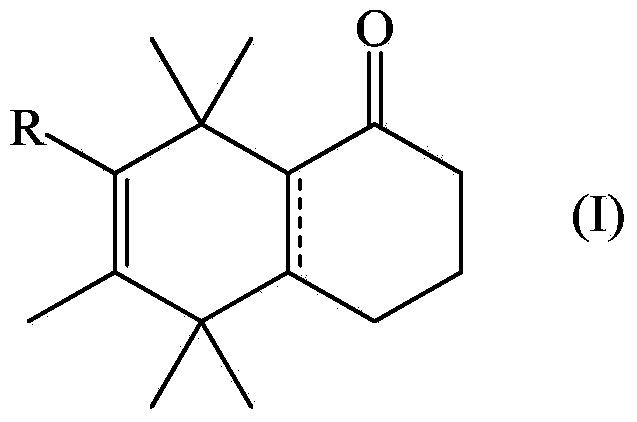
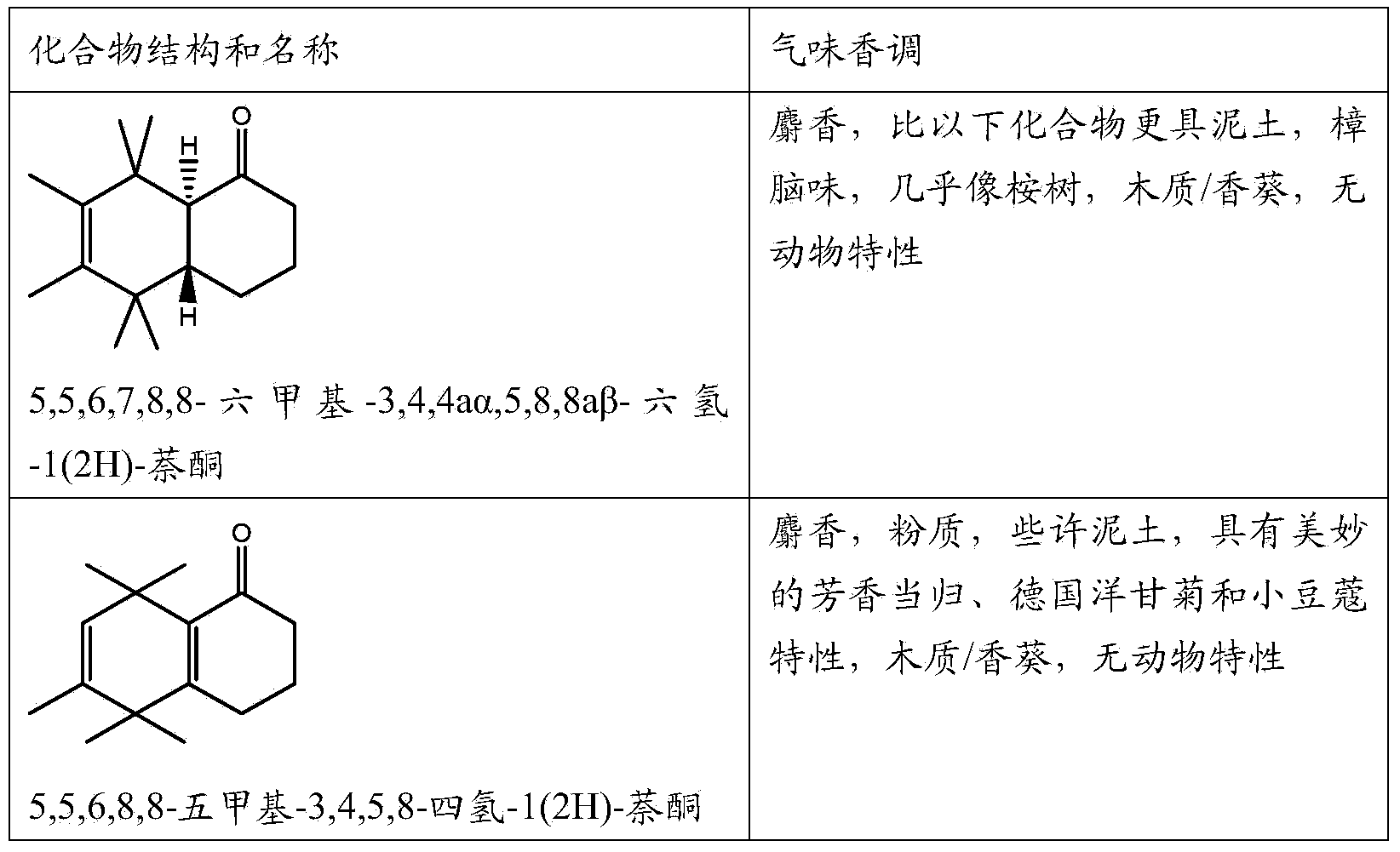
Musk odorant with aromatic notes
A technology of methyl and naphthalenone, applied in the field of flavoring ingredients, penta/hexamethyl-3,4,5,8-tetrahydro-1-naphthone derivatives, can solve the sensory properties that have not been reported or suggested, There are no reports or hints about the application of the compound, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Synthesis of compounds of general formula (I)
[0049] -Methyl 2,3,3,6,6-pentamethyl-4-oxocyclohex-1-enecarboxylate (2a)
[0050] A solution of tBuOK (16.28 g in 70 ml diglyme) and MeI (11.32 ml) were added simultaneously over 1.5 h to 2,6,6-trimethyl-4-oxocyclohexyl-2 cooled to 0°C In a solution of methyl-enecarboxylate (15g, 72.7mmol) in diglyme (80ml). The mixture was stirred at 0 °C for 2 hours, then quenched with 5% HCl. with Et 2 O extract the mixture and water, saturated NH 4 The organic layer was washed with OH solution, water, saturated bicarbonate solution and brine. with MgSO 4 The collected organic layers were dried, and the solvent was distilled off. The crude product was purified by distillation (125 °C, 0.1 mbar) and column chromatography (silica gel, cyclohexane / EtOAc 98 / 2) to give (2a). Yield: 27%.
[0051] -Methyl 2,3,3,5,6,6-hexamethyl-4-oxocyclohex-1-enecarboxylate (2b)
[0052] (2a) (1.0g, 4.38mmol) in THF (5ml) was slowly added to LDA (f...
Embodiment 2
[0075] Preparation of Perfuming Compositions
[0076] A perfuming composition for a body wash was prepared by mixing the following ingredients:
[0077]
[0078]
[0079] *in dipropylene glycol
[0080] 1) (-)-(8R)-8,12-epoxy-13,14,15,16-destetramethyllysium (tetranorlabdane); source: Firmenich SA, Geneva, Switzerland
[0081] 2) Tetrahydro-2-isobutyl-4-methyl-4(2H)-pyranol; source: Firmenich SA, Geneva, Switzerland
[0082] 3) Methyl cis-dihydrojasmonate; source: Firmenich SA, Geneva, Switzerland
[0083] 4) 1-(Octahydro-2,3,8,8-tetramethyl-2-naphthyl)-1-ethanone; source: International Flavors&Fragrances, USA
[0084] 5) Methyl dihydrojasmonate; source: Firmenich SA, Geneva, Switzerland
[0085] 6) 3,3-Dimethyl-5-(2,2,3-trimethyl-3-cyclopenten-1-yl)-4-penten-2-ol; source: Firmenich SA, Switzerland, Geneva
[0086] Adding 4500 parts by weight of 5,5,6,7,8,8-hexamethyl-3,4,5,8-tetrahydro-1(2H)-naphthone to the above composition gave the composition a strong The m...
Embodiment 3
[0089] Preparation of Perfuming Compositions
[0090] A perfuming composition for men's eau de toilette was prepared by mixing the following ingredients:
[0091]
[0092]
[0093] *in dipropylene glycol
[0094] 1) Methyl cis-dihydrojasmonate; source: Firmenich SA, Geneva, Switzerland
[0095] 2) 4 / 3-(4-Hydroxy-4-methylpentyl)-3-cyclohexene-1-carbaldehyde; Source: International Flavors & Fragrances, USA
[0096] 3) 3,3-Dimethyl-5-(2,2,3-trimethyl-3-cyclobasten-1-yl)-4-penten-2-ol; source: Firmenich SA, Switzerland ,Geneva
[0097] The addition of 2000 parts by weight of 5,5,6,7,8,8-hexamethyl-1,4,5,8-tetrahydro-1(2H)-naphthone to the above combination rendered the composition pure The (animal-free) musk-vanilla specific (twist), boosts the aromatic notes, increases the freshness of the original composition and adds a light fruity note.
[0098] When replacing the compound of the present invention and using the same amount of prior art (6RS, 7RS)-5,5,6,7,8,8-hexam...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com