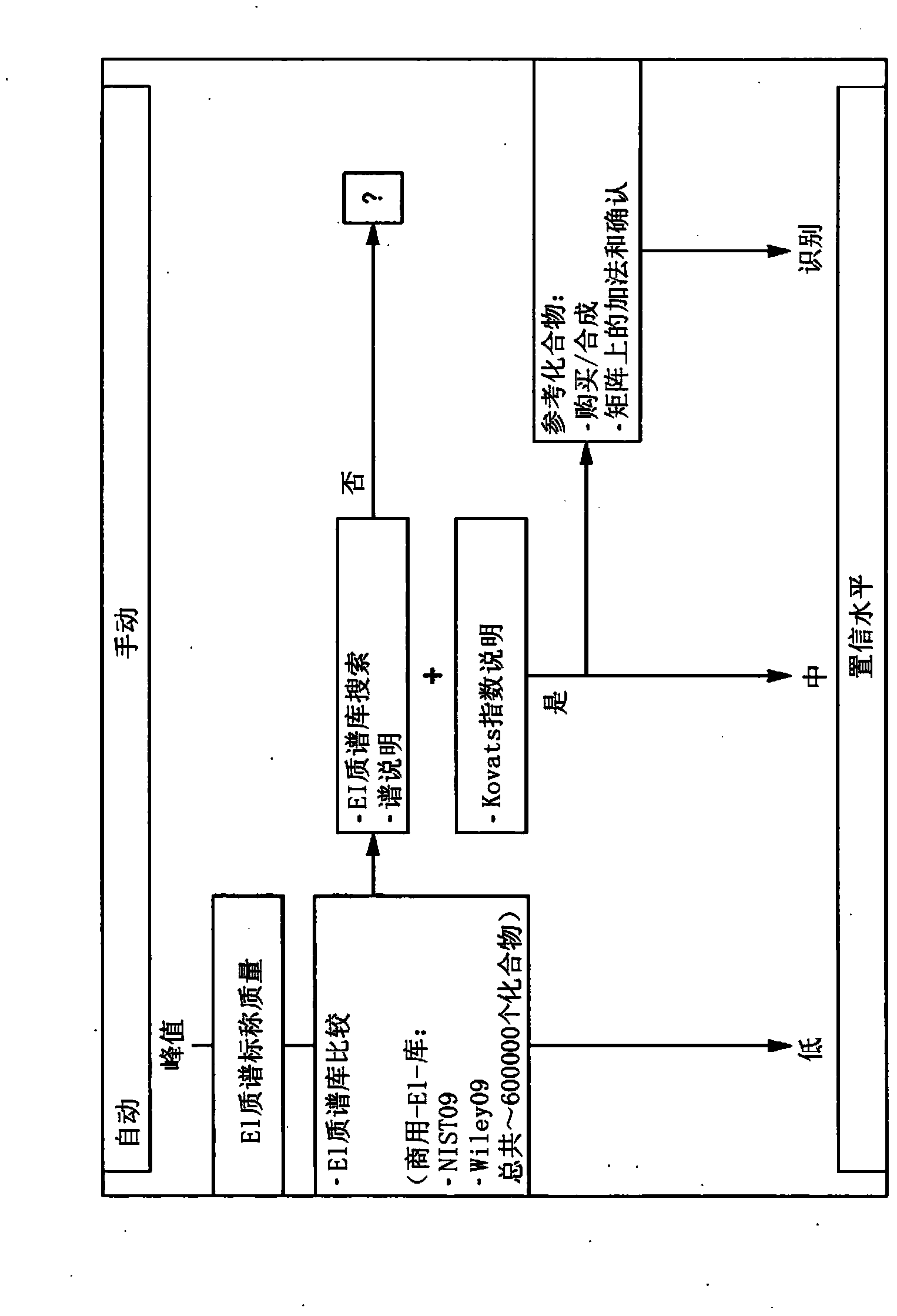
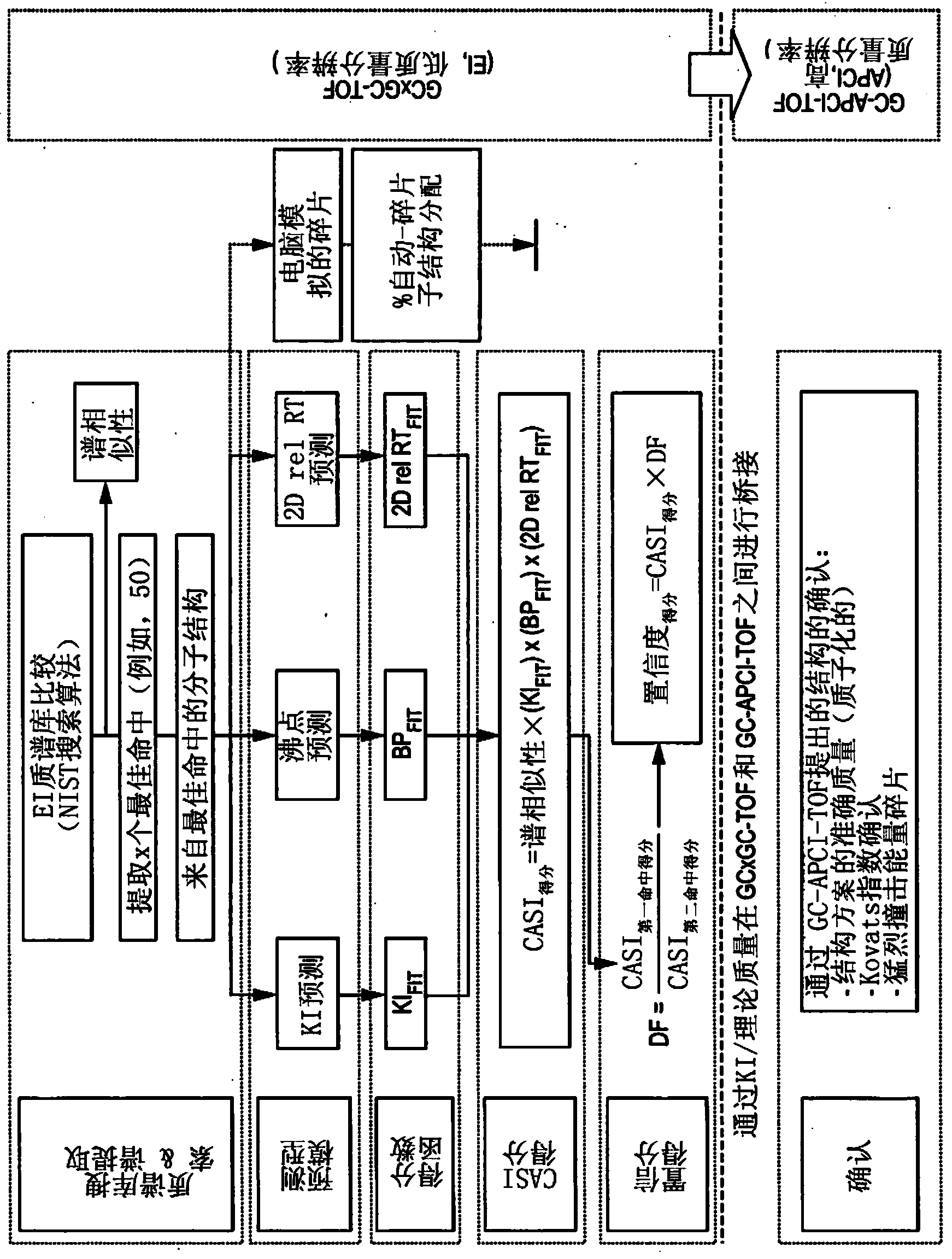
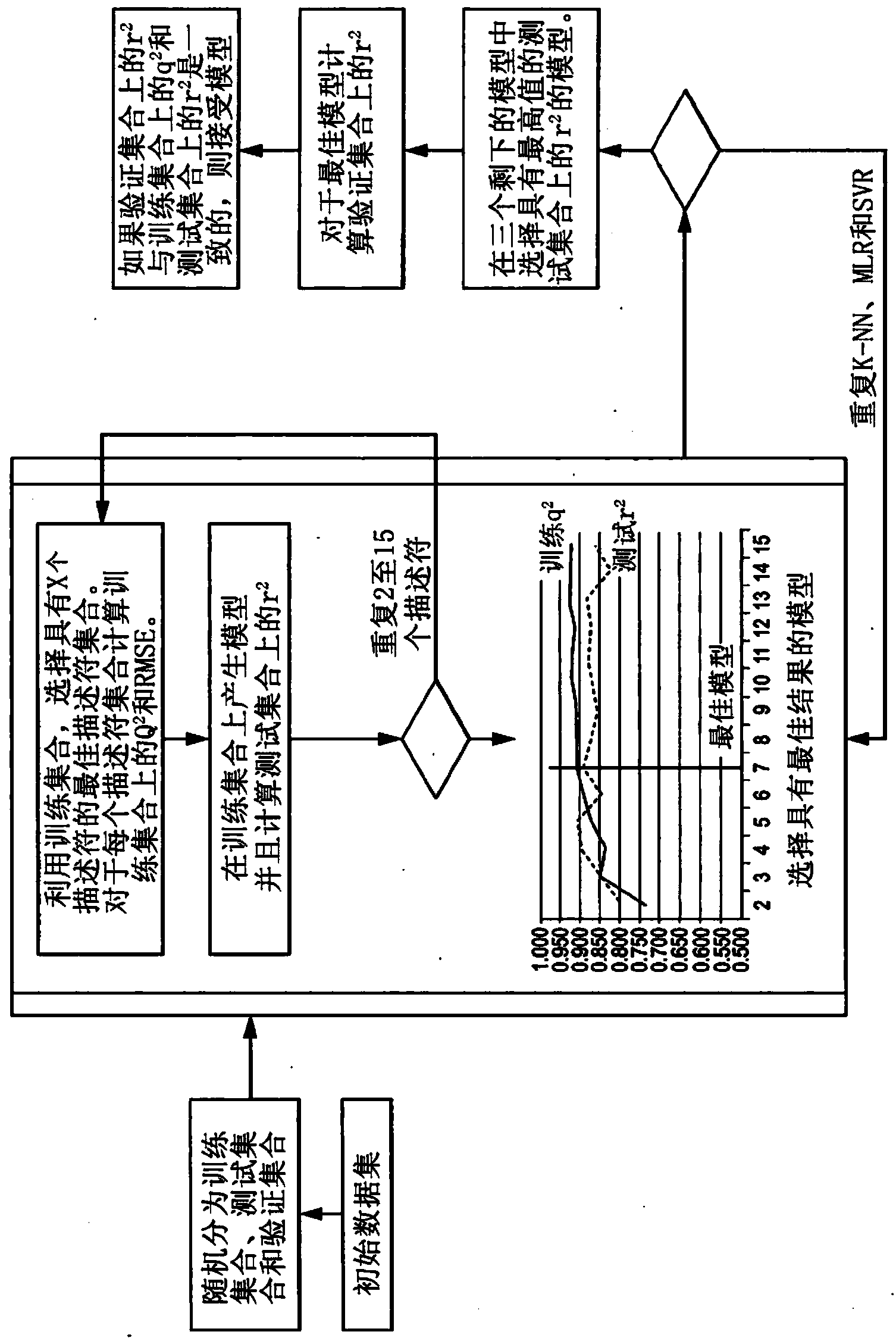
Computer-assisted structure identification
A compound and retention time technology, applied to measuring devices, instruments, discharge tubes, etc., can solve problems such as cost and time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0127] Models used for predictive analysis properties
[0128] Construct all QSPR models for the development of CASI under the same principle. Compounds of known structure are randomly divided into a training set (90 compounds in this example) and a test set (35 compounds in this example). In addition, in this example, 35 different compounds were used as the validation set. Without limitation, 50 to 500 compounds can be used for training. The different distributions of compounds between the collections can be selected for model building. The chemical structure expressed in a computer-readable format is prepared using software known in the prior art (in this case, Pipeline Pilot 8.0.1 (Accelrys Inc., San Diego, California, USA)). During preparation, use a predefined list to desalt from the structure of the compound, retain the largest fragments, deprotonate the main components and protonate the acid, standardize the charge of the functional group, increase hydrogen, and generat...
example 2
[0210] Instruments and analytical methods
[0211] Data generation
[0212] The LECO GC×GC-TOF system Pegasus IV was used for the experiment. The cigarette smoke collected on the glass fiber filter mat is extracted with an organic solution and enhanced with a mixture of several deuterated internal standards and retention time marker compounds. Immediately after the fluid-fluid separation using dichloromethane / water and the derived raw material extract, the cigarette smoke extract is analyzed by BSTFA / TMCS by injecting the extract in the cold head mode into the analysis system. The separation of the composite mixture is performed in a two-dimensional mode using a combination of non-polar / polar analytical columns for the first / second dimension chromatography. The helium as the carrier gas is maintained at a constant flow of 1.0 ml / min. A 30m DB-5ms analytical column with an inner diameter of 0.25 mm and a film thickness of 0.25 μm was used for the first dimension, and a DB-17ht o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com