Catalyst with hydrogenation catalysis effect, preparation method and application of catalyst and hydrocracking method
The technology of a catalyst and an auxiliary agent is applied to a catalyst with hydrogenation catalysis and its preparation and application as well as the fields of hydrocracking, which can solve the problem that the hydrogenation catalytic activity of the catalyst needs to be further improved, and achieve the effect of improving the catalytic activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
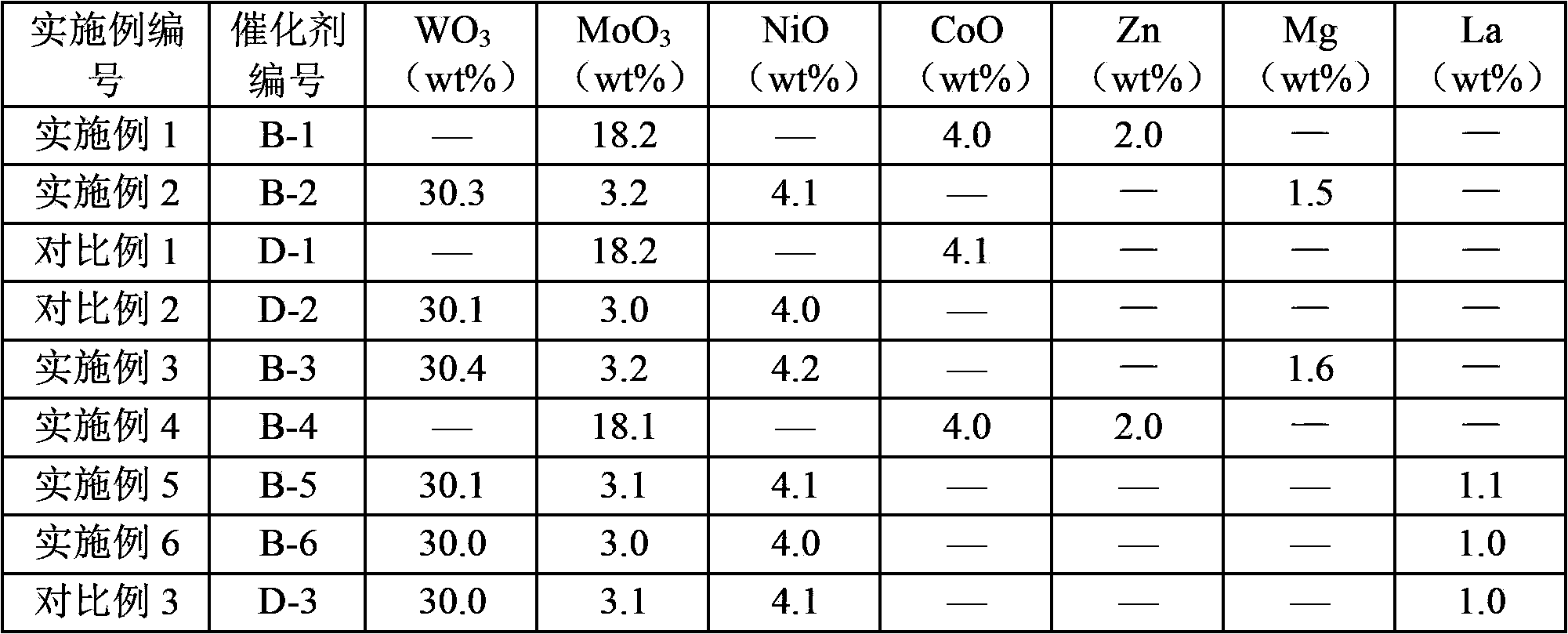
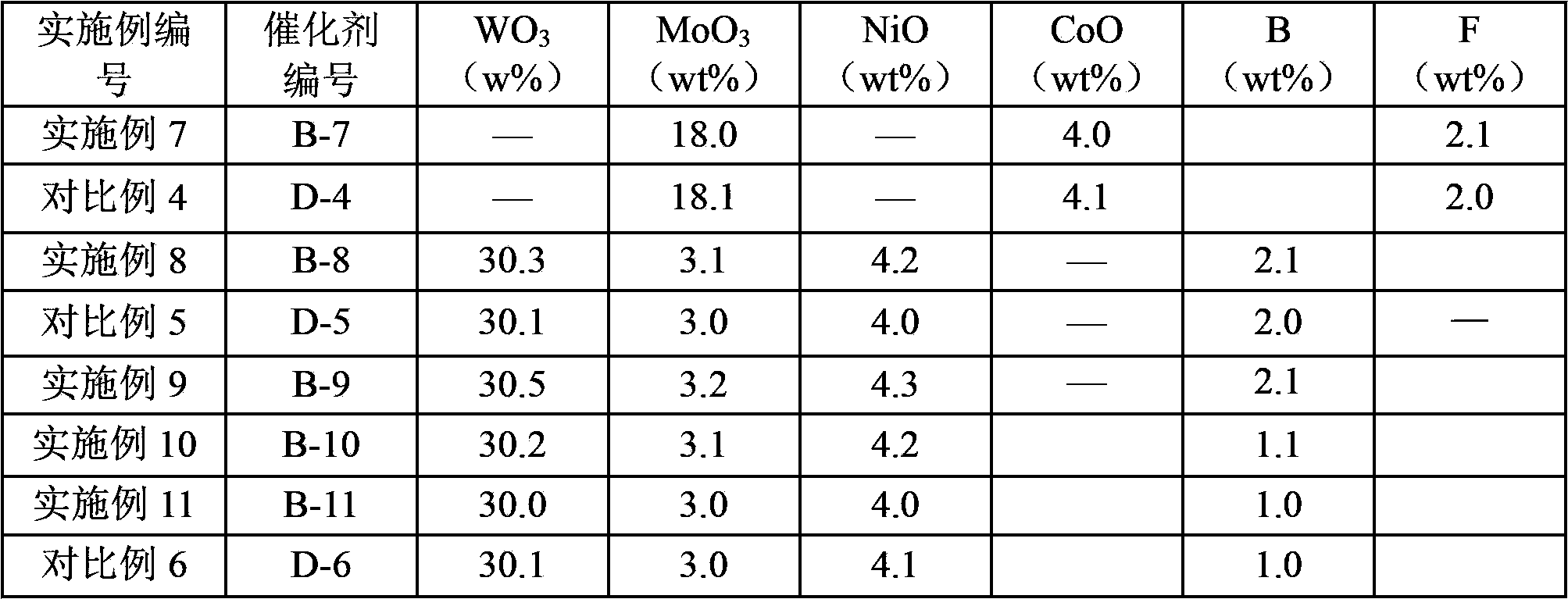
[0027] In the catalyst obtained according to the preparation method of the present invention, the contents of the metal elements of Group VIB, metal elements of Group VIII and elements used as promoters can be properly selected according to the specific application of the catalyst. Generally, the introduction amount of the Group VIB metal element, the Group VIII metal element and the element as an auxiliary agent on the shaped porous carrier is such that, based on the total amount of the catalyst, the shaped The content of the porous carrier can be 30-88.5% by weight, preferably 43-88.5% by weight, more preferably 52-85% by weight; in terms of oxides, the content of Group VIB metal elements can be 10-50% by weight, It is preferably 10-45% by weight, more preferably 12-40% by weight; in terms of oxides, the content of Group VIII metal elements can be 1-10% by weight, preferably 1-7% by weight, more preferably 2- 5% by weight; based on the element, the content of the element as ...
preparation example 1
[0097] (1) Take 200g dry base NaY molecular sieve (commercially purchased from Catalyst Factory of Sinopec Changling Refining and Chemical Company, unit cell constant is The relative crystallinity is 100%, and the specific surface area is 720m 2 / g, sodium oxide content is 13.1% by weight) is placed in the reaction kettle, add 200g of ammonium sulfate and 2000mL of water, raise the temperature to 90°C with stirring, and keep at this temperature for 2 hours. After the reaction, the reaction product was filtered, and the filter cake was washed with water three times to obtain molecular sieve NY-1.
[0098] (2) Take 100 grams of the molecular sieve NY-1 prepared in step (1), put it in a muffle furnace, feed water vapor while raising the temperature, raise the temperature to 550°C, keep the temperature for 2 hours, then cool down and take it out. The obtained product was placed in a reaction kettle, and 1000 mL of an aqueous solution containing sulfuric acid and ammonium sulfate...
preparation example 2
[0102] With 50 grams of REY molecular sieves (commercially purchased from the Catalyst Factory of Sinopec Changling Refining and Chemical Company, the trade name is REHY, and the unit cell constant is The rare earth element content is 3% by weight), 950 grams (on a dry basis) of pseudo-boehmite (commercially purchased from Shandong Aluminum Factory, the trade name is SD powder, and the dry basis content is 69% by weight) and 30 grams of scallop powder Mix and extrude with an extruder into trilobal strips with a circumscribed circle diameter of 1.6 mm. The extruded molded body was dried at 120° C. for 5 hours, followed by firing at 550° C. for 3 hours to obtain a carrier S2. In the carrier S2, the content of macroporous molecular sieve is 5.0% by weight, and the content of alumina is 95.0% by weight.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com