Naphthodithiophene derivative organic electroluminescent material and application thereof
A technology of dithiophene and derivatives, applied in the field of new organic materials, can solve the problem of low hole mobility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
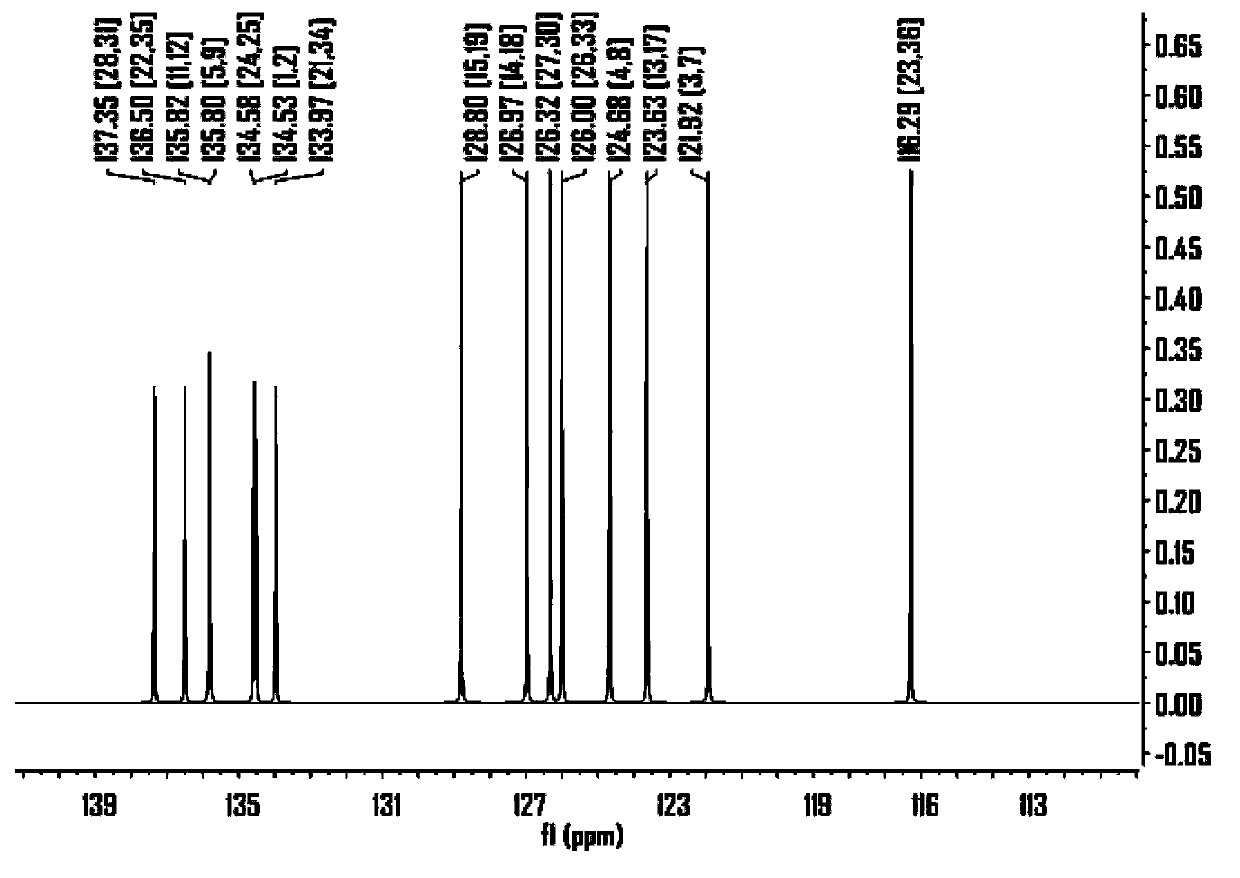
[0119] The synthesis of embodiment 1 compound 1
[0120] (1) The first step
[0121]
[0122] Under the protection of Ar gas, add 12.6g (molecular weight: 240, 0.0526mol) naphtho[2,3-b:6,7-b']dithiophene, 250ml dry THF into a dry reactor, cool to -80 ℃, add dropwise 53ml of t-BuLi (concentration 2.4M, 0.127mol) under stirring, naturally raise the temperature to -10°C under stirring, then cool down to -50°C again, add dropwise 35ml (molecular weight 326, specific gravity 1.20, 0.129 mol) of tributyltin chloride, stirred overnight. The next day, the reaction was terminated with 200ml of sodium bicarbonate (concentration 0.1M), the product was extracted with dichloromethane, and the product was separated by column chromatography (eluent: petroleum ether / triethylamine=95 / 5) to obtain 31g of dichloromethane. Yellow solid product, yield 71%, molecular weight 818.
[0123] (2) The second step
[0124]
[0125] 9.8g of ditin compound synthesized in the first step (molecular ...
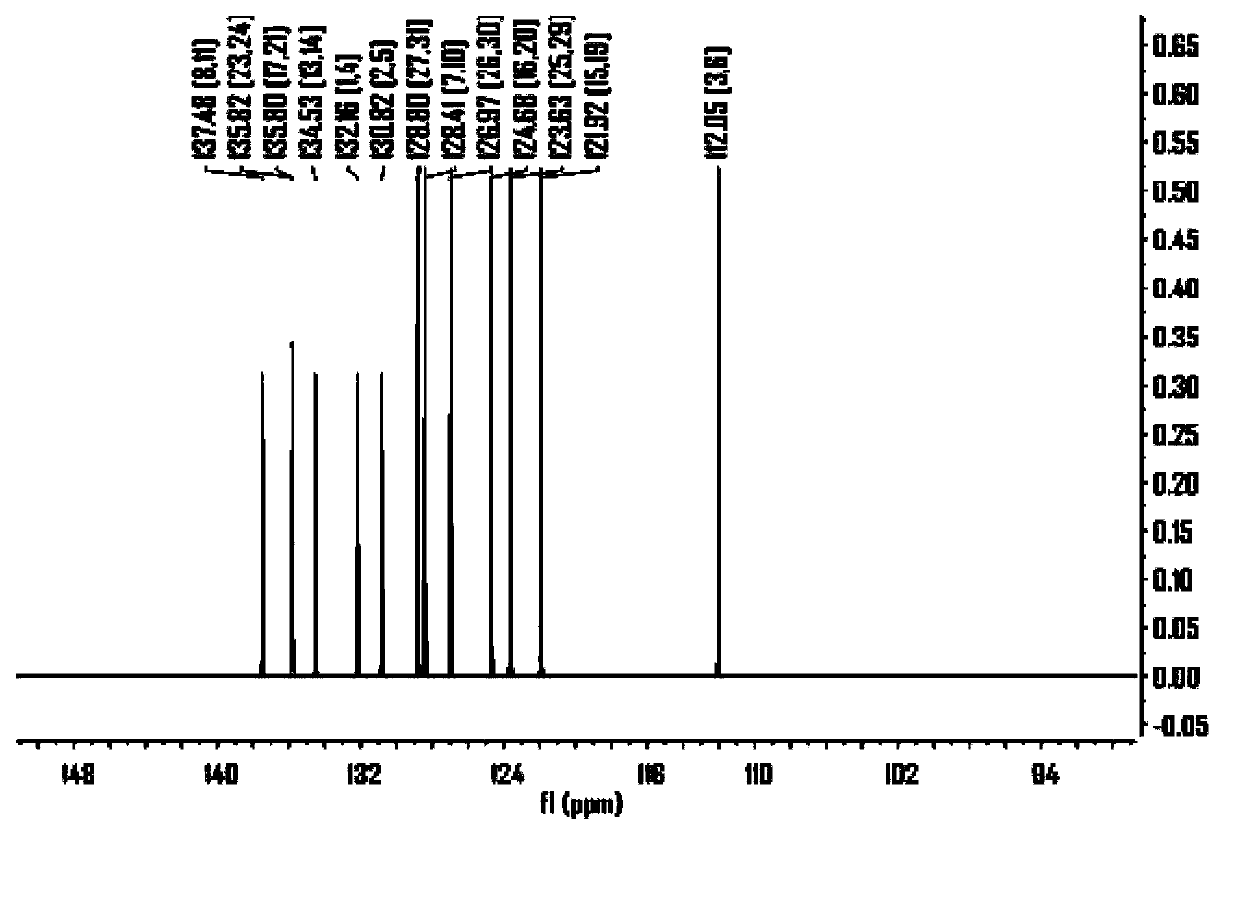
Embodiment 2
[0127] The synthesis of embodiment 2 compound 2
[0128]
[0129] The synthesis procedure is the same as the second step in Example 1, except that one of the raw materials, 5-(thiophen-2-yl)-2-bromothiophene, is changed to 5-(2-naphthyl)-2-bromothiophene, The product was obtained as a yellow solid.
[0130] Product MS (m / e): 656, elemental analysis (C 42 h 24 S 4 ): theoretical value C: 76.79%, H: 3.68%, S: 19.53%; measured value C: 76.82%, H: 3.73%, S: 19.45%.
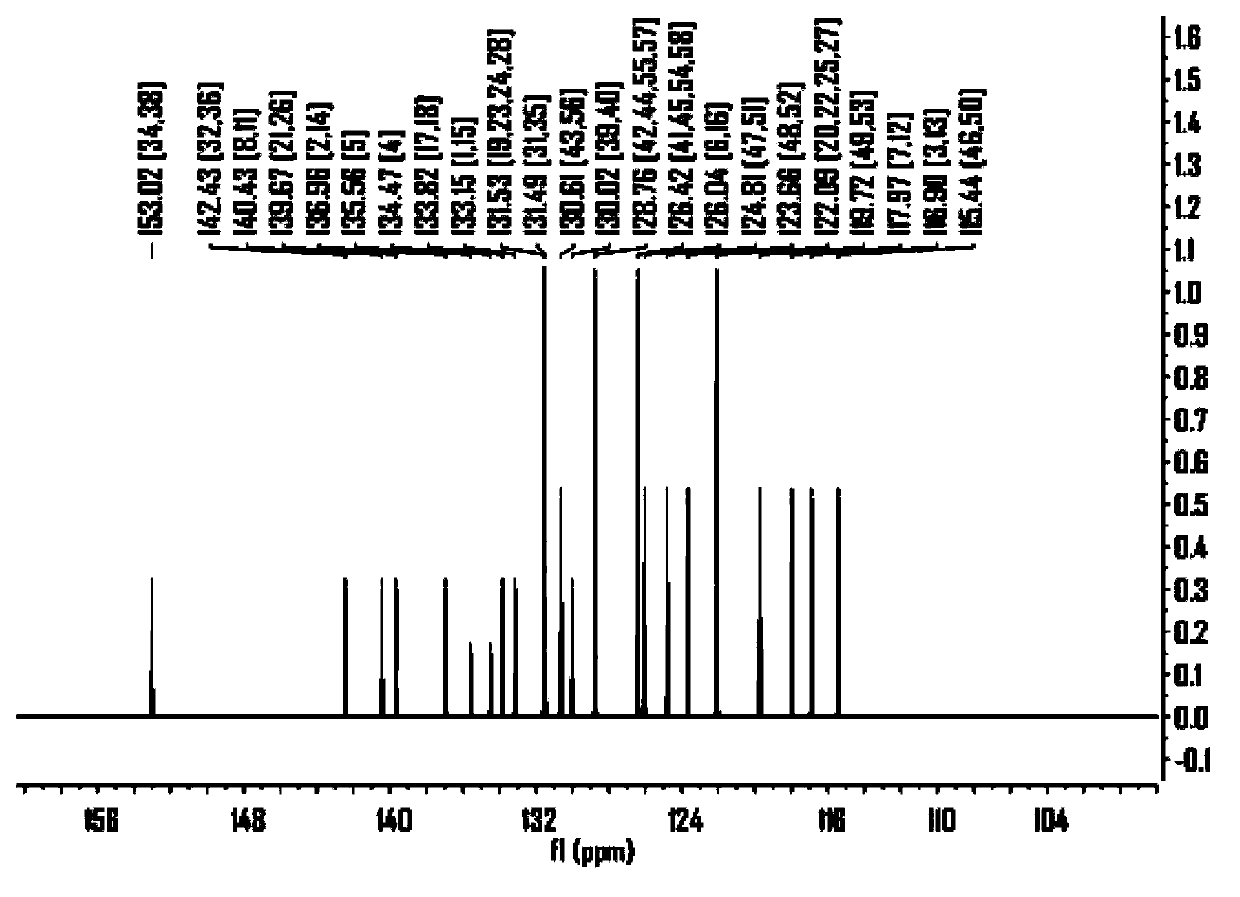
Embodiment 3
[0131] The synthesis of embodiment 3 compound 3
[0132]
[0133] The synthesis procedure is the same as the second step in Example 1, except that one of the raw materials, 5-(thiophen-2-yl)-2-bromothiophene, is changed to 4-(benzothiophen-2-yl)bromobenzene, The product was obtained as a pale yellow solid.
[0134] Product MS (m / e): 656, elemental analysis (C 42 h 24 S 4 ): theoretical value C: 76.79%, H: 3.68%, S: 19.53%; measured value C: 76.72%, H: 3.65%, S: 19.63%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com