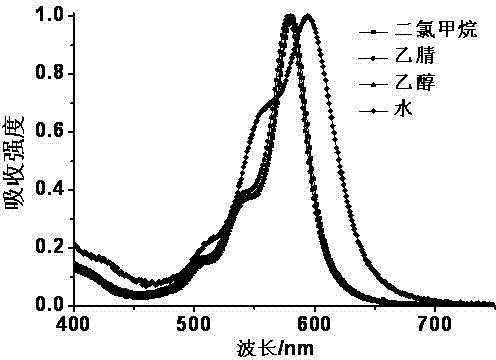
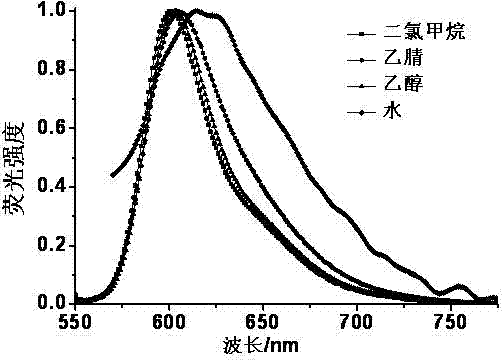
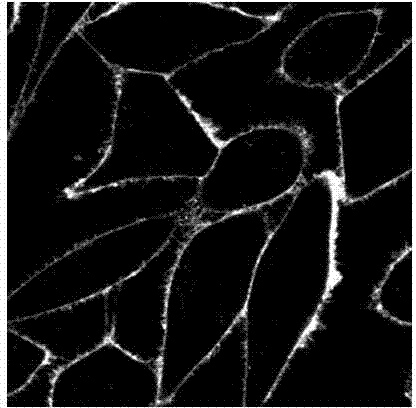
Fluorescent probe for marking and tracking cytoplasmic membranes and preparation method of fluorescent probe
A fluorescent probe and cytoplasmic membrane technology, applied in the field of fluorescent probes and their preparation, can solve the problems of difficult observation of the activity mechanism by scientists, poor stability of cell membrane labeling, cumbersome synthesis and preparation process, etc. The effect of improving the signal-to-noise ratio
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022]
[0023] Add 324 mg of fluoroborate dipyrrole and 420 mg of carbazole aldehyde into a two-necked reaction flask, then add 8.5 mg of piperidine as a catalyst, and 4? After removing toluene, silica gel column separation (eluent: dichloromethane:methanol=30:1) gave 234mg of purple solid, yield 40%.
[0024]
[0025] Dissolve 234 mg of the product obtained in the previous step in dichloromethane, add 568 mg of iodomethane, react at 25 ° C for 24 hours, filter with suction, and retain the filter cake to obtain 262 mg of purple-black solid fluorescent probe A-1, with a yield of 90%.
[0026]
[0027] Dissolve 586mg of fluorescent probe A-1 in methanol, add 940mg of potassium bromide, and stir at 50°C for 24h. Press and spin dry to obtain 646 mg purple-black solid fluorescent probe B-1 with a yield of 95%.
Embodiment 2
[0029]
[0030] Add 482 mg of fluoroborate dipyrrole and 584 mg of carbazole aldehyde into a two-necked reaction flask, then add 25.5 mg of piperidine as a catalyst, and 4? Spin to dry under reduced pressure, remove toluene, and separate on silica gel column (eluent: dichloromethane:methanol=20:1) to obtain 824 mg of green solid with a yield of 80%.
[0031]
[0032] Dissolve 824 mg of the product obtained in the previous step in dichloromethane, add 269 mg of acrolein and an appropriate amount of HCl, react at 25°C for 24 hours, filter with suction, and retain the filter cake to obtain 923 mg of dark green solid fluorescent probe A-2 with a yield of 95 %.
[0033]
[0034] Dissolve 1214mg of fluorescent probe A-2 in methanol, 2300mg of potassium periodate, and stir at 50°C for 24h. After the reaction is completed, the reaction solution is spin-dried under reduced pressure, dissolved in dichloromethane, filtered, and the filtrate is kept and reduced again. Press and ...
Embodiment 3
[0036]
[0037] Add 376 mg of fluoroborate dipyrrole and 672 mg of carbazole aldehyde into a two-necked reaction flask, then add 25.5 mg of piperidine as a catalyst, and 4? Spin to dry under reduced pressure, remove toluene, and separate on silica gel column (eluent: dichloromethane:methanol=15:1) to obtain 760 mg of green solid with a yield of 75%.
[0038]
[0039] Dissolve 760 mg of the product obtained in the previous step in dichloromethane, add 488 mg of propane sultone and an appropriate amount of HCl, react at 25°C for 24 hours, add an appropriate amount of ether, filter with suction, and retain the filter cake to obtain 898 mg of dark green solid fluorescent probe A -3, 90% yield.
[0040]
[0041]Dissolve 1,330 mg of fluorescent probe A-3 in methanol, add 1,030 mg of sodium bromide, and stir at 50°C for 24 hours. After the reaction is complete, the reaction solution is spin-dried under reduced pressure, dissolved in dichloromethane, filtered, and the filtrat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com