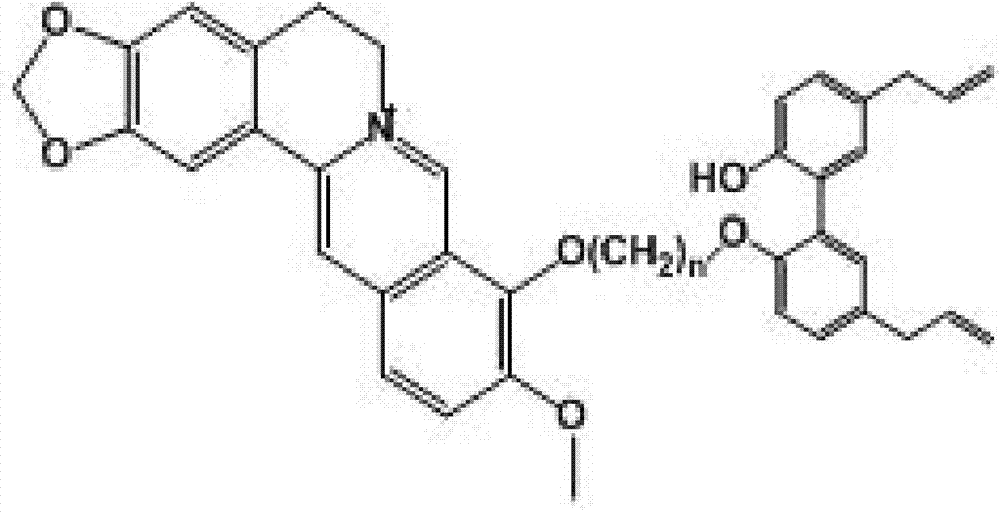
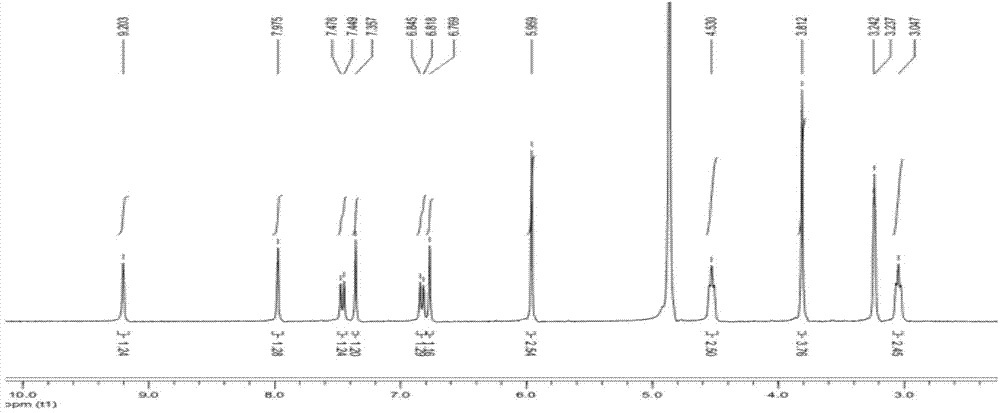
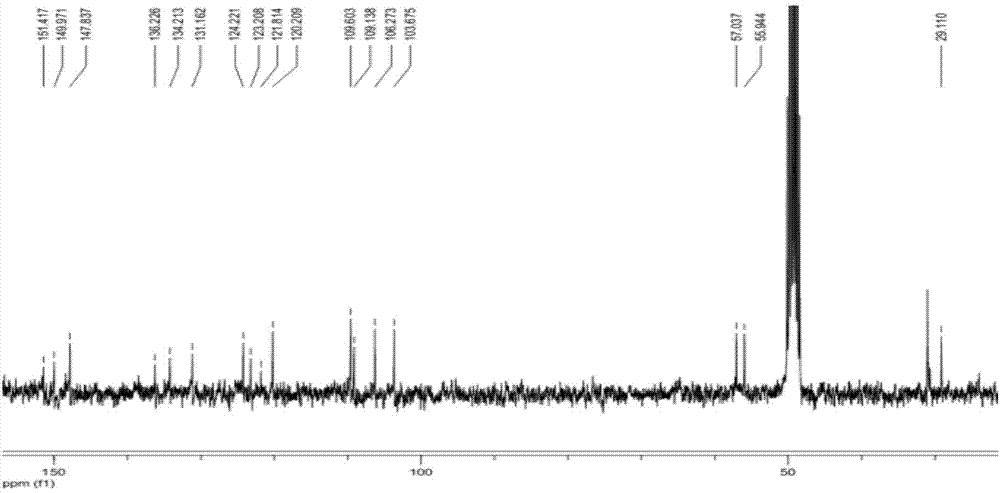
Poly-nuclear molecular compound and preparation method and application thereof
A technology of multinuclear molecules and compounds, applied in the fields of medicinal chemistry and pharmacotherapeutics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0124] An embodiment of the multinuclear molecular compound of the present invention, the multinuclear molecular compound described in this embodiment is prepared by the following method:
[0125] (1a) Synthesis of berberine derivatives: Weigh 1 part by weight of berberine, add 150 parts by weight of acetonitrile, heat to boiling at 85°C, add X(CH 2 ) n Y40 parts by weight, reflux for 3 hours, concentrate the reaction solution to 50 parts, cool and crystallize, filter, wash the crystals with an appropriate amount of acetonitrile, combine the washing liquid with the filtrate, recover acetonitrile, dissolve the residue with 30 parts by weight of methanol, cool and crystallize, filter, Wash the crystals with methanol, and merge with the above-mentioned crystals to obtain a sample yield of 56.34%, a berberine derivative with a purity of more than 95%, wherein the X(CH 2 ) n In Y, both X and Y are Br, n=2, and the obtained berberine derivative is berberine-9-oxyethyl bromide, w...
Embodiment 2
[0147] An embodiment of the multinuclear molecular compound of the present invention, the multinuclear molecular compound described in this embodiment is prepared by the following method:
[0148] (1a) Synthesis of berberine derivatives: weigh 1 part by weight of berberine, add 200 parts by weight of acetonitrile, heat to boiling at 85°C, add X(CH 2 ) n 20 parts by weight of Y, reflux for 3 hours, concentrate the reaction solution to 100 parts, cool and crystallize, filter, wash the crystals with an appropriate amount of acetonitrile, combine the washing liquid with the filtrate, recover acetonitrile, dissolve the residue with 30 parts by weight of methanol, cool and crystallize, filter, Wash the crystals with methanol, and merge with the above-mentioned crystals to obtain a sample yield of 56.34%, a berberine derivative with a purity of more than 95%, wherein the X(CH 2 ) n In Y, both X and Y are Cl, n=3;
[0149](2a) Mix 1.2 parts by weight of the berberine derivative o...
Embodiment 3
[0152] An embodiment of the multinuclear molecular compound of the present invention, the multinuclear molecular compound described in this embodiment is prepared by the following method:
[0153] (1a) Synthesis of berberine derivatives: Weigh 1 part by weight of berberine, add 160 parts by weight of acetonitrile, heat to boiling at 85°C, add X(CH 2 ) n Y30 parts by weight, reflux for 3 hours, concentrate the reaction solution to 80 parts, cool and crystallize, filter, wash the crystals with an appropriate amount of acetonitrile, combine the washing liquid with the filtrate, recover acetonitrile, dissolve the residue with 30 parts by weight of methanol, cool and crystallize, filter, Wash the crystals with methanol, and merge with the above-mentioned crystals to obtain a sample yield of 56.34%, a berberine derivative with a purity of more than 95%, wherein the X(CH 2 ) n In Y, both X and Y are S, n=4;
[0154] (2a) Mix 1.2 parts by weight of the berberine derivative obtain...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com