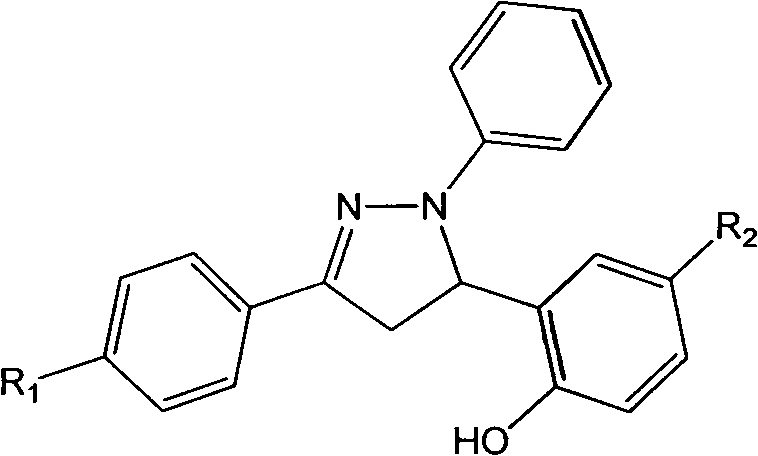
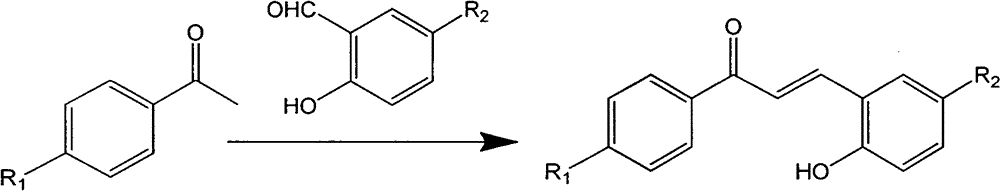
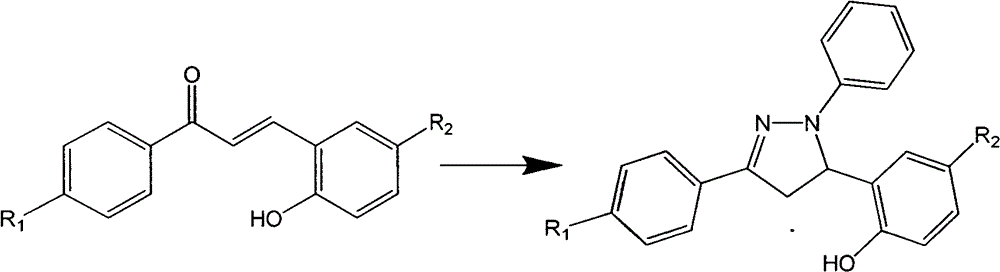
A class of pyrazoline derivatives prepared from salicylaldehyde and preparation method thereof
A technology of pyrazolines and salicylaldehyde, which is applied in the field of pyrazoline derivatives and their preparation, can solve the problems of increased salt-forming property and increased possibility, and achieve the effect of increasing the possibility of salt-forming
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1: Preparation of 2-(3-(4-fluorophenyl)-1-phenyl-4,5-dihydro-1H-pyrazol-5-yl)phenol (compound 1)
[0023]
[0024] Take 5.0mmol of p-fluoroacetophenone and 5.25mmol of salicylaldehyde and add them to a 50ml round bottom flask, add 20ml of absolute ethanol, stir magnetically for 10min under ice bath conditions to mix evenly, slowly drop 10ml of 40% NaOH solution, Magnetic stirring, react in ice bath for 0.5h, then move to normal temperature for 5h (TLC detects the progress of the reaction), after the reaction, adjust the pH to acidic (pH value is 6.0), the product is precipitated in solid form, suction filtered, and washed with cold ethanol 3 times (3ml each time), dry, and the product is recrystallized with a mixture of ethanol and acetone (volume ratio ethanol:acetone=10:1); add 2.0mmol of the above product and 2.1mmol of phenylhydrazine to a 50ml flask, and dissolve it in 20ml of anhydrous Ethanol was heated to 80°C to dissolve, and refluxed at 80°C for 2h ...
Embodiment 2
[0025] Example 2: Preparation of 2-(3-(4-chlorophenyl)-1-phenyl-4,5-dihydro-1H-pyrazol-5-yl)phenol (compound 2)
[0026]
[0027] The preparation method is the same as in Example 1. The p-fluoroacetophenone in Example 1 was replaced by p-chloroacetophenone to obtain the target compound in light yellow powder form. Yellowpowder, Yield54%, mp: 54-56℃. 1 HNMR (CDCl 3 , 300MHz) δ: 3.09-3.17 (dd, J 1 = 10.5Hz,J 2 =6.6Hz, 1H), 3.87-3.93 (dd, J 1 = 10.5Hz,J 2 =7.5Hz, 1H), 5.62-5.66(m, 1H), 6.77-6.79(t, J=4.5Hz, 1H), 6.84-6.88(m, 2H), 6.97-6.98(d, J=4.8Hz, 2H), 7.20-7.31(m, 4H), 7.55-7.57(d, J=5.4Hz, 2H), 7.95-7.97(d, J=5.7Hz, 2H), 10.06(m, 1H).MS(ESI ): 349.10 (C 21 h 18 ClN 2 O, [M+H] + ).Anal.CalcdforC 21 h 17 ClN 2 O: C, 72.31; H, 4.91; Cl, 10.16; N, 8.03; O, 4.59. Found: C, 72.13; H, 4.91; N, 8.04.
Embodiment 3
[0028] Example 3: Preparation of 2-(3-(4-bromophenyl)-1-phenyl-4,5-dihydro-1H-pyrazol-5-yl)phenol (compound 3)
[0029]
[0030] The preparation method is the same as in Example 1. The p-fluoroacetophenone in Example 1 was replaced by p-bromoacetophenone to obtain the target compound in light yellow powder form. Yellowpowder, Yield52%, mp: 65-68℃. 1 HNMR (CDCl 3 , 300MHz) δ: 3.09-3.16 (dd, J 1 = 10.5Hz,J 2 =6.6Hz, 1H), 3.87-3.93 (dd, J 1 = 10.5Hz,J 2 =7.5Hz, 1H), 5.61-5.65(m, 1H), 6.76-6.78(t, J=4.8Hz, 1H), 6.82-6.85(m, 2H), 6.90-6.91(d, J=4.8Hz, 2H), 7.19-7.26(m, 4H), 7.59-7.61(d, J=5.4Hz, 2H), 7.66-7.68(d, J=5.4Hz, 2H), 10.04(m, 1H).MS(ESI ): 393.05 (C 21 h 18 BrN 2O, [M+H] + ).Anal.CalcdforC 21 h 17 BrN 2 O: C, 64.13; H, 4.36; Br, 20.32; N, 7.12; O, 4.07. Found: C, 64.01; H, 4.36; N, 7.13.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com