Method for preparing artemisinin by using residue obtained through extraction of artemisinin from sweet wormwood herb
An artemisinin and residue technology, applied in the direction of organic chemistry and the like, can solve the problems of low-cost, large-scale preparation of artemisinin, extraction of active components of artemisinin, and high cost of photoreaction equipment, and achieves simple structure and low manufacturing cost. , the effect of improving the preparation efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
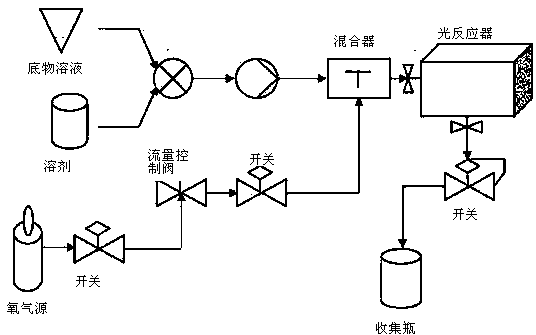
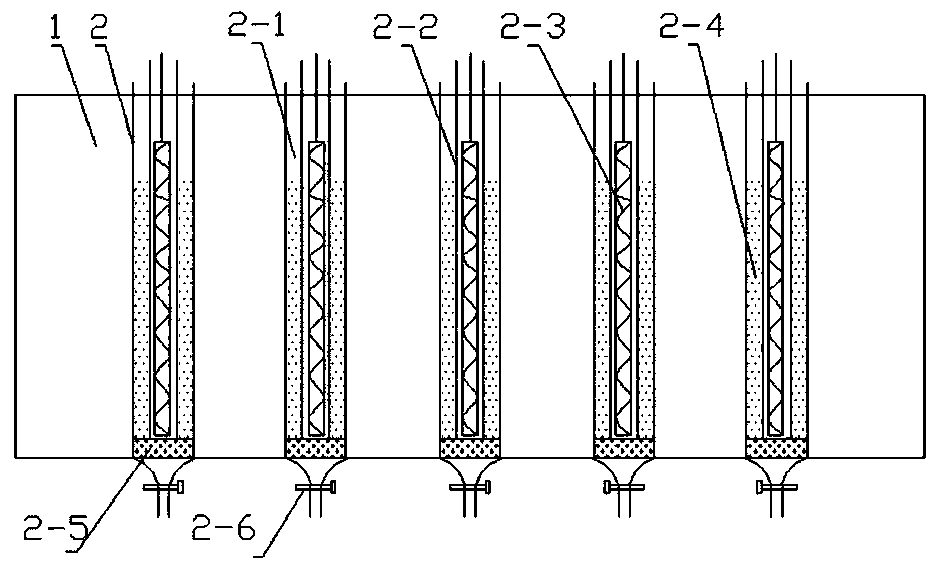
[0052] The photo-oxidation reaction equipment used in this embodiment consists of a constant temperature tank 1 and ten 6L photoreactors 2, and the light source used is an LED light source.
[0053] 1) Add 100Kg of residue to 1m 3 In the enamel reaction kettle, pour 0.5m 3 ethyl acetate, stirred at room temperature for 4 hours, repeated extraction 3 times, the extraction temperature was 50 o C, filtered, the filtrate was concentrated, and the residue was 60Kg in total. The residue was sent to HPLC to detect the contents of artemisinic acid and dihydroartemisinic acid, and the measured contents of artemisinic acid and dihydroartemisinic acid were 5% and 10%, respectively.
[0054] 2) Put 60Kg residue obtained in step 1) into 1m 3 In the enamel reaction kettle, pour 0.5m 3 10 L of hydrazine hydrate with a concentration of 80% was added to the reactor, and the temperature of the reactor was lowered to 0°C, and 20 L of 30% hydrogen peroxide was slowly added to the reactor, and...
Embodiment 2
[0062] The photo-oxidation reaction equipment used in this embodiment consists of a constant temperature tank 1 and six 6L photoreactors, and the light source used is an LED light source.
[0063] 1) Add 50Kg of residue to 1m 3 Into the enamel reaction kettle, then pour 0.3m 3 ethyl acetate, stirred at room temperature for 5 hours, repeated extraction 2 times, the extraction temperature was 10 o C, filtered, and the filtrate was concentrated, and the residue was 28Kg in total. The residue was sent to HPLC to detect the contents of artemisinic acid and dihydroartemisinic acid, and the contents of artemisinic acid and dihydroartemisinic acid were measured to be 4.5% and 10.3%, respectively.
[0064] 2) Put the 28Kg residue obtained in step 1) into 1m 3 In the enamel reaction kettle, pour 0.3m 3 THF, stirred, and 5 L of 80% concentration of hydrazine hydrate was added to the reactor, the temperature of the reactor was lowered to 0°C, and 10 L of 30% hydrogen peroxide was slow...
Embodiment 3
[0068] The photo-oxidation reaction equipment used in this embodiment consists of a constant temperature tank 1 and 20 6L photoreactors, and the light source used is an LED light source.
[0069] 1) Add 200Kg of residue to 2m 3 In the enamel reaction kettle, pour 1m 3 ethyl acetate, stirred at room temperature for 5 hours, repeated extraction 4 times, the extraction temperature was 100 o C, filtered, and the filtrate was concentrated, and the residue was 129Kg in total. The residue was sent to HPLC to detect the content of artemisinic acid and dihydroartemisinic acid, and the content of artemisinic acid and dihydroartemisinic acid was measured to be 5.2% and 10.7% respectively;
[0070] 2) Put the 129Kg residue obtained in step 1) into 2m 3 In the enamel reaction kettle, pour 1m 3 20L of hydrazine hydrate with a concentration of 80% was added to the reactor, the temperature of the reactor was lowered to 0°C, and 40L of hydrogen peroxide with a mass concentration of 30% was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com