A tumor marker and its application in the preparation of colorectal cancer diagnostic reagents
A technology of colorectal cancer and diagnostic reagents, applied in the field of serum/tissue tumor markers, can solve problems such as low sensitivity, failure to achieve diagnosis, and inaccurate indicators of pancreatic cancer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Embodiment 1 sample collection and processing
[0049] Tissue samples from 7 patients with colon cancer and 10 samples from the control group were clinically collected, and the clinical data of the patients were systematically collected.
Embodiment 2
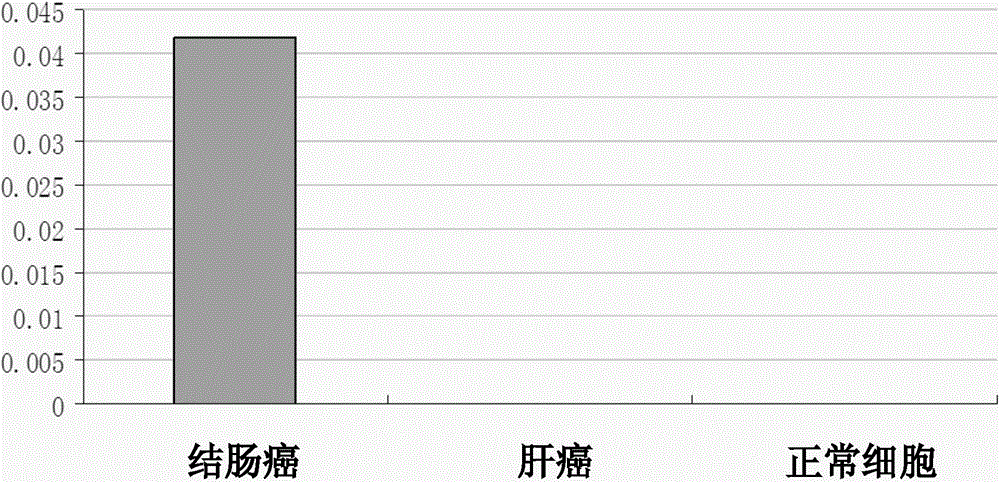
[0050] Detection of mRNA in embodiment two colon cancer and normal cells
[0051] 1. Cell total RNA extraction, use chloroform extraction method to extract the total RNA of cells, the specific steps are as follows:
[0052] (1) Take the colon cancer, liver cancer and normal cells in the medium dish, add 1ml Trizol reagent to lyse the tissue, repeatedly blow with a gun or shake vigorously to lyse the cells;
[0053] (2) Transfer the Trizol lysate of the above cells into an EP tube, and place it at room temperature 15-30°C for 5 minutes; (3) Add 0.2 ml of chloroform to the above EP tube according to the amount of 0.2 ml of chloroform per 1 ml of TRIZOL, and cover Put the cap on the EP tube, shake it vigorously in your hand for 15 seconds, let it stand at room temperature (15°C-30°C) for 2-3 minutes, then centrifuge at 12000g (4°C) for 30 minutes;
[0054] (4) After centrifugation, take the upper aqueous phase and put it in a new EP tube, add 1 volume of isopropanol according to...
Embodiment 3
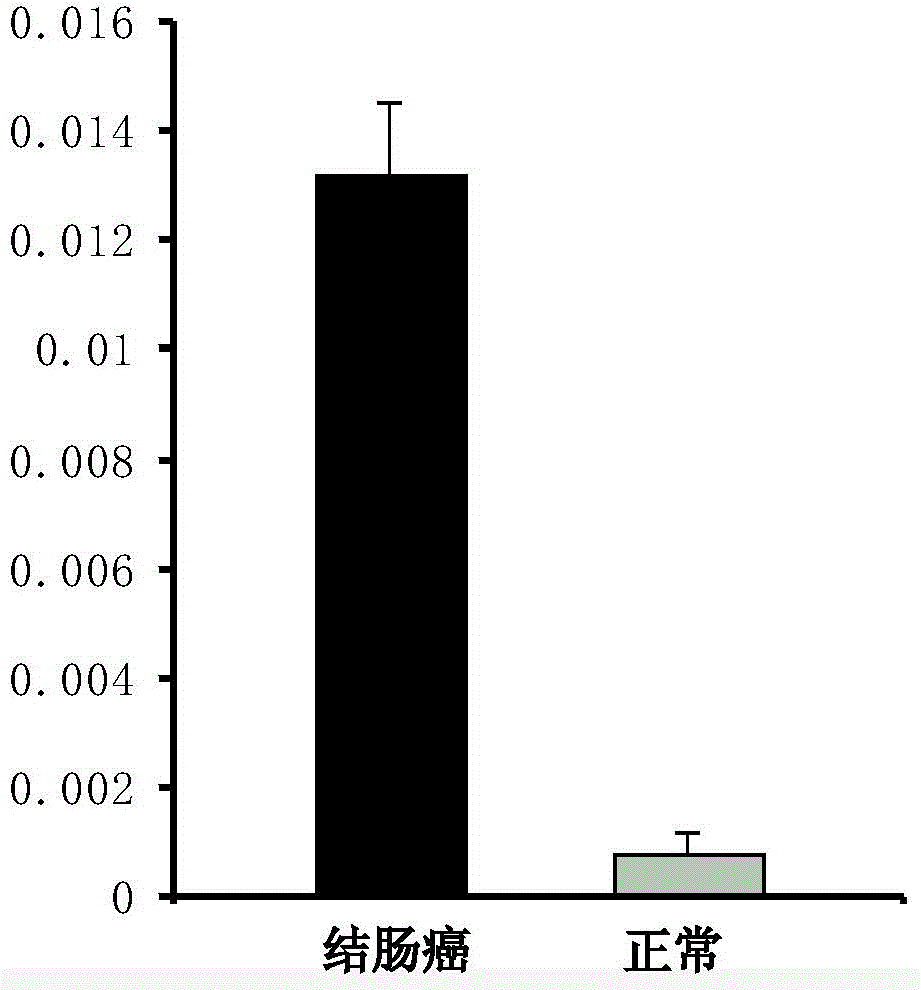
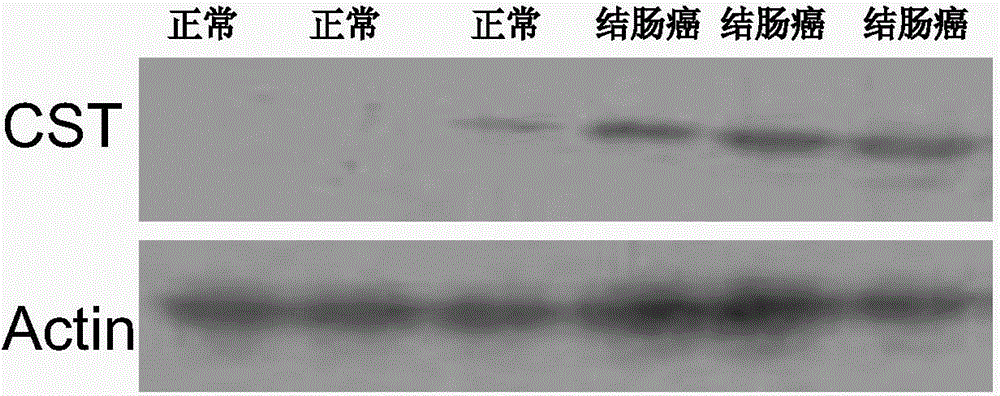
[0070] Example 3 CST1, CST4, CST2mRNA qRT-PCR experiments in tissues
[0071] 1. According to the cell results, CST1, CST4, and CST2 were further verified by qRT-PCR.
[0072] 2. Extraction of total RNA from tissue samples, using the chloroform extraction method to extract total RNA from tissue samples, the specific steps are as follows:
[0073] (9) Take the patient's frozen colon tissue sample, knock out a tissue block with a size of about 2mm×2mm×2mm, add 1ml Trizol reagent to lyse the tissue, and repeatedly use a gun to blow or shake vigorously to lyse the tissue; (10) The above tissue Transfer the Trizol lysate into an EP tube and place it at room temperature 15-30°C for 5 minutes;
[0074] (11) In the above EP tube, add 0.2ml chloroform for every 1ml TRIZOL, cover the EP tube cap, vibrate vigorously in your hand for 15 seconds, and place it at room temperature (15°C-30°C) for 2-3 minutes Afterwards, centrifuge at 12000g (4°C) for 30 minutes;
[0075] (12) After centri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com