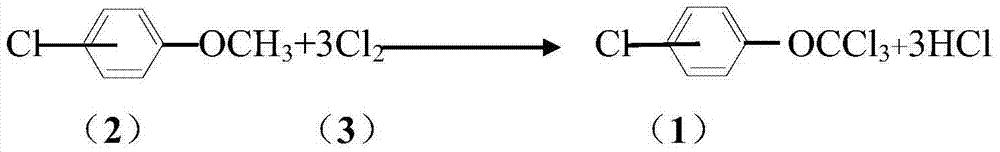
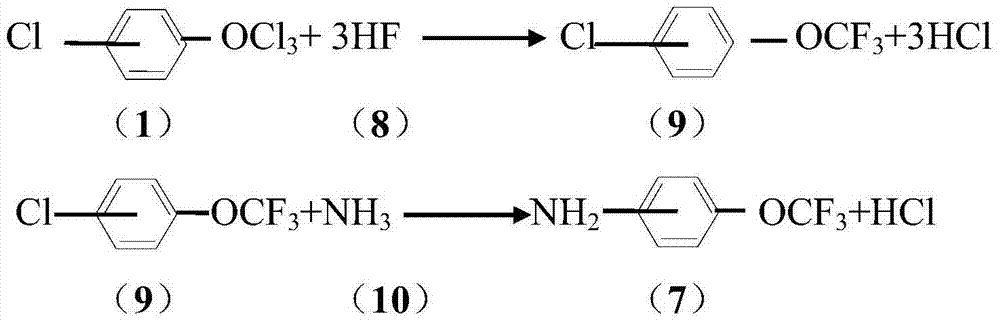
Method for synthesizing trichlorine methoxyl chlorobenzene and trichlorine methoxyl phenylamine
A technology of trichloromethoxychlorobenzene and chlorotrichloromethoxybenzene, which can be applied in chemical instruments and methods, preparation of organic compounds, preparation of aminohydroxy compounds, etc. Yield decline and other problems, to achieve high industrial value, cheap and easy to obtain raw materials, less by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] In a 500mL reaction flask, add p-trifluoromethylbenzene (200g, 145ml), 2 grams of dimethyl azobisisobutyrate and 2 grams of phosphorus trichloride in sequence, raise the temperature to 60°C, and turn on the mercury lamp for the reaction The solution was illuminated, and chlorine gas was introduced into the bottle at a rate of 20ml / s. Simultaneously, 80g (0.56mol) of p-chloroanisole was added dropwise, and the dripping was completed in 1 hour. After the dropwise addition, continue to maintain the reaction at 70-80°C. The end point of the reaction is tracked and detected by GC. The mass content of dichlorobenzyl <0.5% is the end point of the reaction. At this time, the reaction time is 5 hours. Stop feeding chlorine gas, and then carry out air purging. The solvent in the reaction solution was removed by distillation (ceramic corrugated packing) to obtain 135 grams of the target product (4-chlorotrichloromethoxybenzene), with a content of 95.2% and a yield of 93%.
[0056]...
Embodiment 2
[0059] Into a 500 mL reaction flask, add p-trifluoromethylbenzene (400 g, 285 ml), 2 g of dimethyl azobisisobutyrate and 2 g of phosphorus trichloride in sequence, and the rest of the operations are the same as in Example 1. After removing the solvent from the reaction solution, 136 g of the target product (4-chlorotrichloromethoxybenzene) was obtained, with a content of 95.4% and a yield of 93.9%.
Embodiment 3
[0061] Into a 500 mL reaction flask, add p-trifluoromethylbenzene (480 g, 345 ml), 2 g of dimethyl azobisisobutyrate and 2 g of phosphorus trichloride in sequence, and the rest of the operations are the same as in Example 1. After removing the solvent from the reaction solution, 136 g of the target product (4-chlorotrichloromethoxybenzene) was obtained, with a content of 95.5% and a yield of 94.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com