Method for preparing methylacrolein through oxidization of isobutene or tert-butyl alcohol
A technology of methacrolein and tert-butanol, applied in chemical instruments and methods, preparation of carbon-based compounds, preparation of organic compounds, etc., can solve problems such as low yield of methacrolein
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
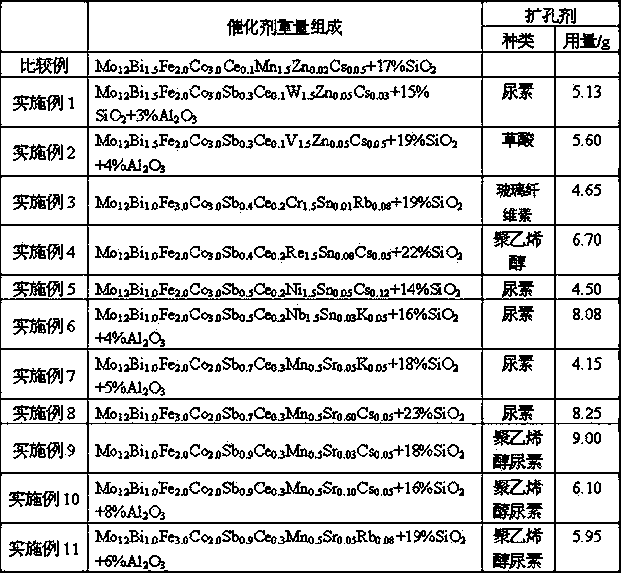
[0034] 100 g (NH 4 ) 6 Mo 7 o 24 4H 2 O was added to 100 grams of warm water at 70°C, stirred to dissolve it completely, and 89.4 grams of 40% (wt.) silica sol, 35.8 grams of 20% aluminum sol and 19.1 grams of (NH 4 ) 6 h 5 [H 2 (WO 4 ) 6 ] to make material A.
[0035] 38.5 g Fe(NO 3 ) 3 9H 2 O was added to 150 g of 70°C hot water, stirred and dissolved, and then 34.3 g of Bi(NO 3 ) 3 ·5H 2 O, 41.2 g Co(NO 3 ) 2 ·6H 2 O, 1.88 g Ce(NO 3 ) 3 ·3H 2 O, 0.7 g Zn(NO 3 ) 2 ·6H 2 O, 0.27 g CsNO 3 Make material B after stirring and dissolving.
[0036] Dissolve 2.1 g of antimony trioxide in 30 g of 10% by mass dilute hydrochloric acid aqueous solution to obtain solution C. Add solutions B and C respectively to solution A under stirring to form a catalyst slurry, add 5.13 g of urea, and stir and age at 80°C for 2 hours, dry the slurry at 120°C to remove most of the water, and extrude to obtain A cylindrical object of φ3.5x3.5mm, and then calcined at hi...
Embodiment 2
[0039] 100 g (NH 4 ) 6 Mo 7 o 24 4H 2 O was added to 100 grams of warm water at 70 ° C, stirred to dissolve it all, and 78.9 grams of 40% (wt.) silica sol, 47.7 grams of 20% aluminum sol and 8.24 grams of NH 4 VO 3 Material A is made.
[0040] 38.5 g Fe(NO 3 ) 3 9H 2 O was added to 150 g of 70°C hot water, stirred and dissolved, and then 34.3 g of Bi(NO 3 ) 3 ·5H 2 O, 41.2 g Co(NO 3 ) 2 ·6H 2 O, 2.1 g Ce(NO 3 ) 3 ·3H 2 O, 0.7 g Zn(NO 3 ) 2 ·6H 2 O, 0.27 g CsNO 3 Make material B after stirring and dissolving.
[0041] Dissolve 2.1 g of antimony trioxide in 30 g of 10% by mass dilute hydrochloric acid aqueous solution to obtain solution C. Add solutions B and C respectively to solution A under stirring to form a catalyst slurry, add 5.60 g of oxalic acid, and stir and age at 80°C for 2 hours, dry the slurry at 100°C to remove most of the water, and extrude to obtain A cylindrical object of φ3.5x3.5mm, and then calcined at high temperature to obtain...
Embodiment 3~11
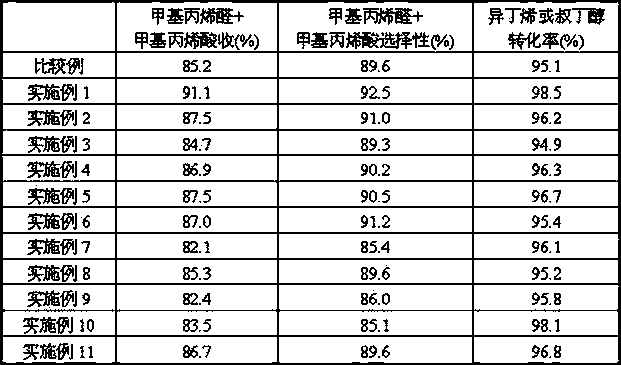
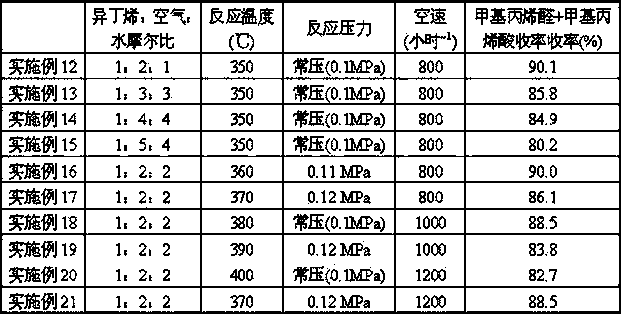
[0044] Prepare catalyst by each step of [embodiment 2], concrete result is listed in table 1. Under the evaluation condition identical with [Example 2], reaction result is listed in Table 2.
[0045]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com