The synthetic method of 4-amino-2-chloro-3-nitropyridine
A technology of nitropyridine and synthesis method, applied in the synthesis field of 4-amino-2-chloro-3-nitropyridine, can solve the problem of inability to separate by column chromatography, changing mobile phase ratio, poor separation effect, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0030] Embodiment 1 A kind of synthetic method of 4-amino-2-chloro-3-nitropyridine
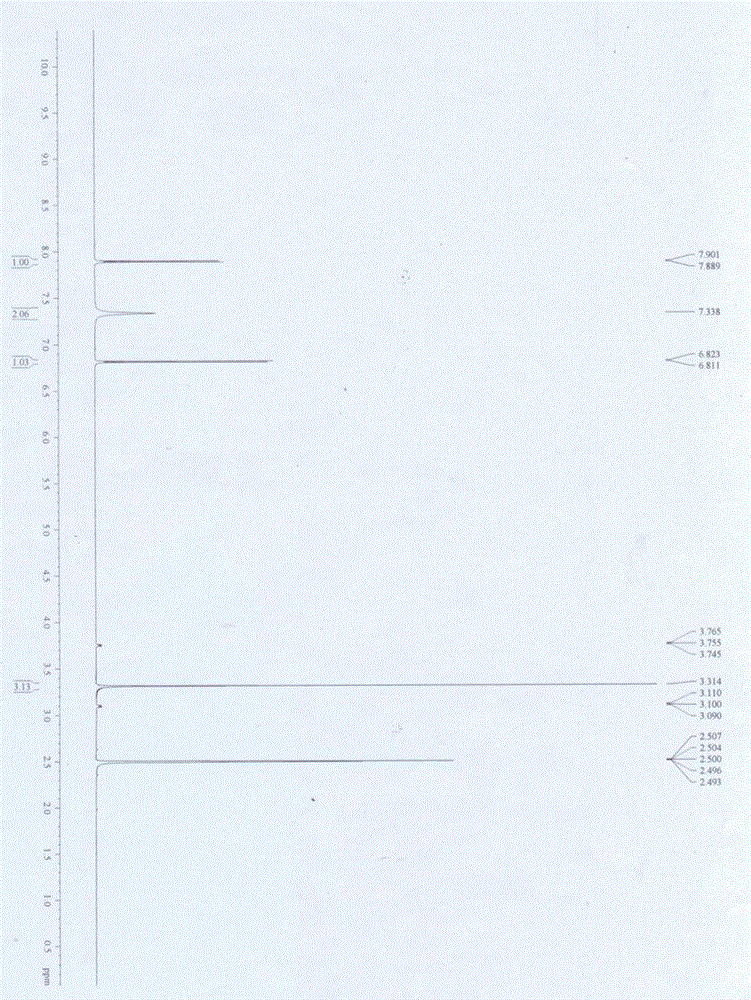
[0031] A kind of synthetic method of 4-amino-2-chloro-3-nitropyridine adopts 2-chloro-4-aminopyridine as raw material, and 65% nitric acid and concentrated sulfuric acid are used as mixed acid to carry out nitration reaction to prepare 4-amino-2- Chloro-3-nitropyridine generates 4-amino-2-chloro-5-nitropyridine at the same time, and the corresponding pure product is obtained after recrystallization and purification. The process is as follows:
[0032] .
[0033] Its synthetic method is carried out according to the following sequence of steps:
[0034] 1) Dissolve 200g of 2-chloro-4-aminopyridine in 1200mL of concentrated sulfuric acid at 0°C, add dropwise 1000mL of 65% nitric acid, after the addition, react at 15°C for 2h, then pour into ice In water, stir at 0°C, add NH 3 Regulate the pH to be 3, separate out white powdery solid I, filter;
[0035] 2) Dissolve the white powdery solid I ...
Embodiment 2
[0039] Embodiment 2 A kind of synthetic method of 4-amino-2-chloro-3-nitropyridine
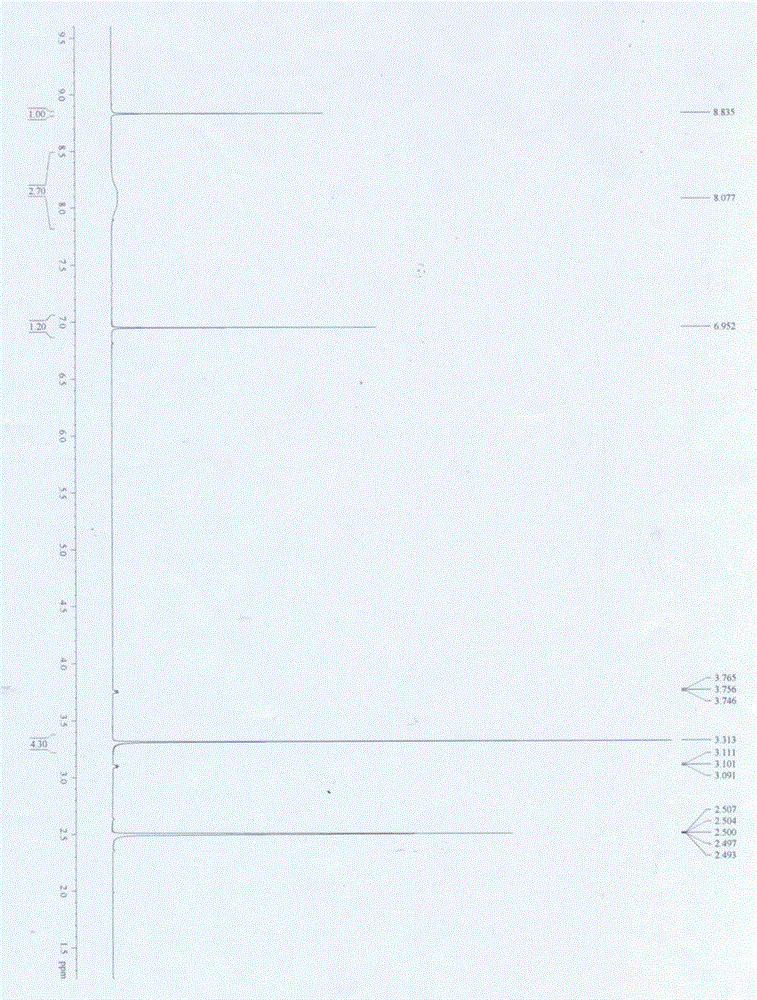
[0040] A kind of synthetic method of 4-amino-2-chloro-3-nitropyridine adopts 2-chloro-4-aminopyridine as raw material, and 65% nitric acid and concentrated sulfuric acid are used as mixed acid to carry out nitration reaction to prepare 4-amino-2- Chloro-3-nitropyridine generates 4-amino-2-chloro-5-nitropyridine at the same time, and the corresponding pure product is obtained after recrystallization and purification. The process is as follows:
[0041] .
[0042] Its synthetic method is carried out according to the following sequence of steps:
[0043] 1) At 0°C, dissolve 200g of 2-chloro-4-aminopyridine in 1800mL of concentrated sulfuric acid, add dropwise 1000mL of 65% concentrated nitric acid, after the dropwise addition, react at 20°C for 2h, then pour into Stir in ice water at 0°C, add NH 3 Regulate pH to be 3, separate out white powdery solid I, filter; Then,
[0044] 2) Dissolve th...
Embodiment 3-124
[0048] The synthetic method of embodiment 3-124-amino-2-chloro-3-nitropyridine
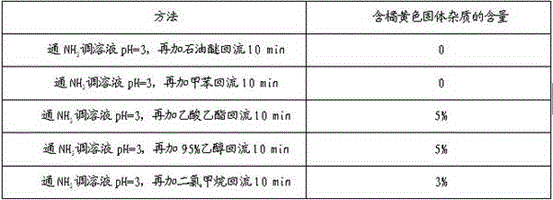
[0049] Embodiment 3-7 is respectively a kind of synthetic method of 4-amino-2-chloro-3-nitropyridine, and the difference between them and Example 1 is that the technical parameters involved in the synthetic method are different, specifically as follows Show:
[0050]
[0051]
[0052] Note: Isomer purity and yield refer to the total purity and yield of 4-amino-2-chloro-3-nitropyridine and 4-amino-2-chloro-5-nitropyridine.
[0053] Embodiments 8-12 are respectively a kind of synthetic method of 4-amino-2-chloro-3-nitropyridine, and they differ from Example 2 only in that the technical parameters involved in the synthetic method are different, specifically as follows Show:
[0054]
[0055]
[0056] Note: Isomer purity and yield refer to the total purity and yield of 4-amino-2-chloro-3-nitropyridine and 4-amino-2-chloro-5-nitropyridine.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com