Assay for detection of jc virus dna
A JC virus, virus technology, applied in the field of nucleic acid detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Example 1: DNA extraction from cerebrospinal fluid (CSF)
[0063] Materials and Method
[0064] -Modified the QIAamp MinElute Virus Spin Kit (Cat#57704, QIAGEN) protocol for processing human CSF samples. The following buffers are used for DNA extraction
[0065] -Buffer AW2 was prepared by adding 30 mL of ethanol (96%-100%) to a bottle containing 13 mL of buffer AW2 concentrate and mixing well. Store the buffer at ambient temperature.
[0066] -Prepare QIAGEN protease by adding 1.4 mL of buffer AVE to a bottle of lyophilized QIAGEN protease and mixing gently. Store the protease at 2°C-8°C.
[0067] -Carrier RNA solution (1 μg / μL): Prepared by adding 310 μL of buffer AVE to a tube of freeze-dried carrier RNA to prepare a 1 μg / μL solution and mixing by pulse vortexing. The carrier RNA was stored at -20+10°C and not subjected to more than three freeze-thaw cycles. The final concentration of carrier RNA in buffer AL was 5.6 μg / mL. For example, for n samples, [(1.1)×(5.6)×(n)] ...
Embodiment 2
[0076] Example 2: Real-time PCR assay for JCVDNA quantification
[0077] Materials and Method
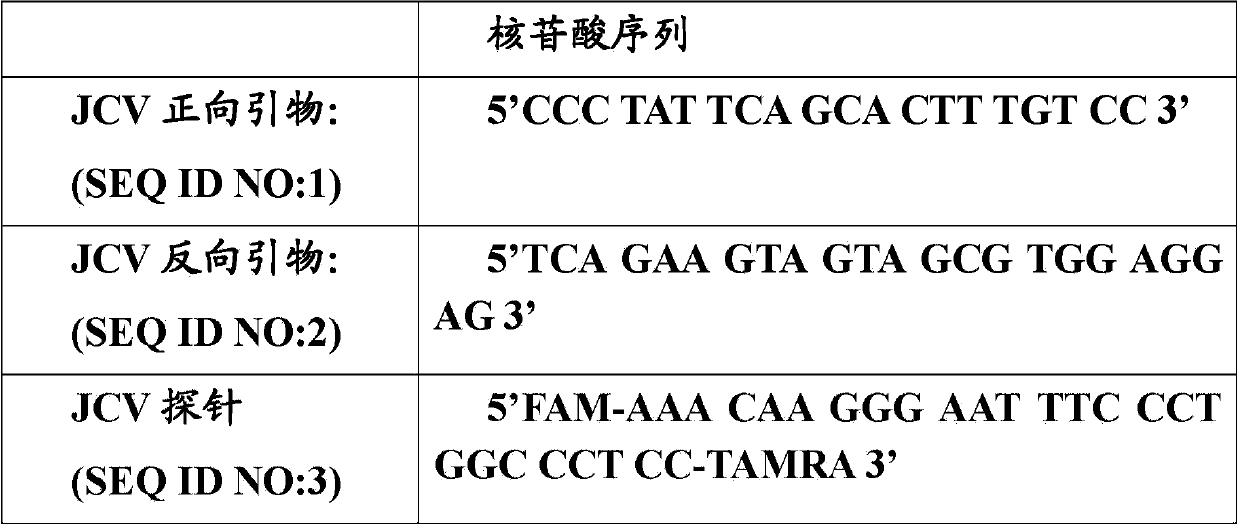
[0078] Primers and probes are designed for conserved regions of the T antigen gene of the JC virus genome, and BLAST searches are performed to ensure cross-reactivity. The sequences of primers and probes are as follows:
[0079]
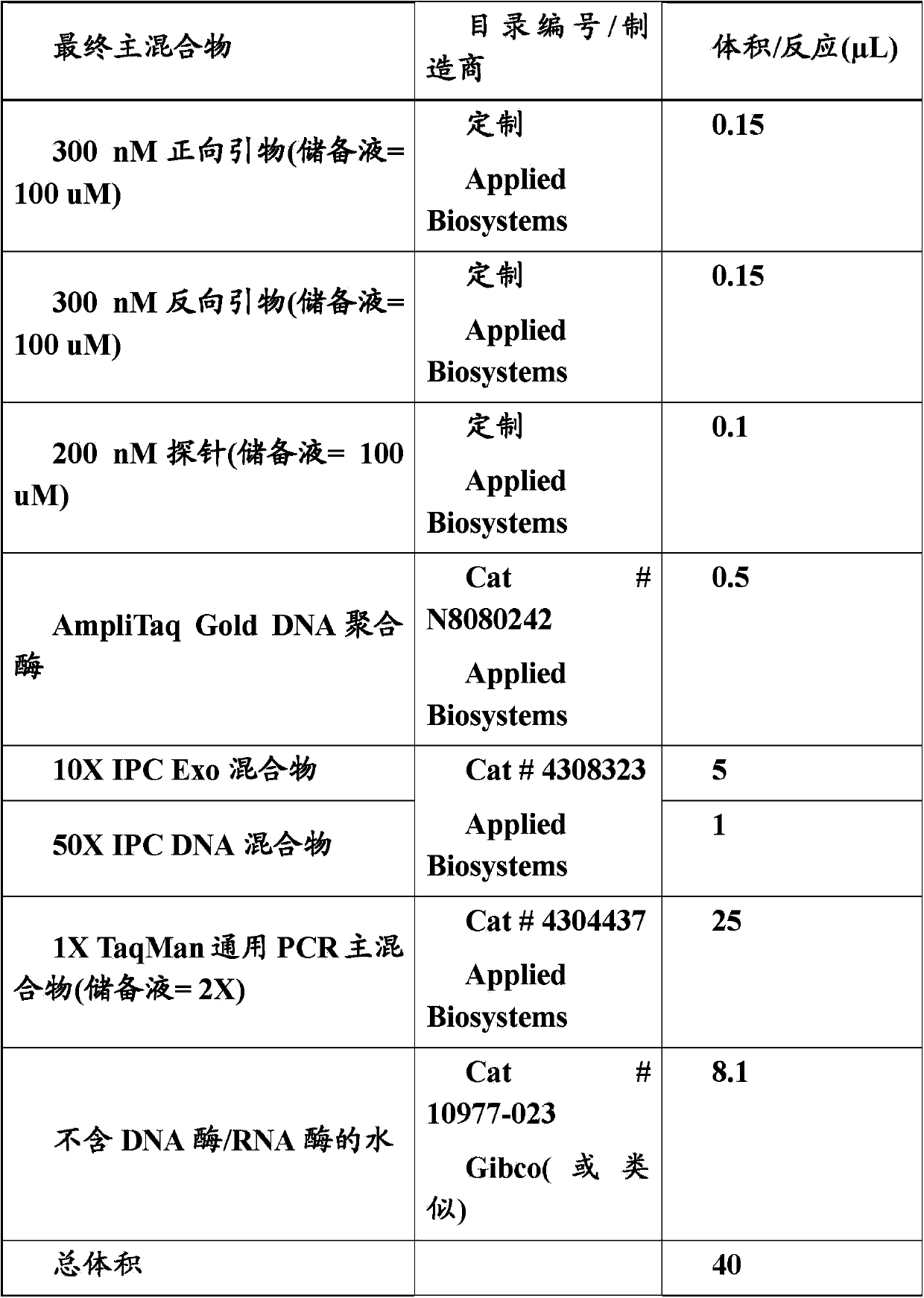
[0080] ABI7900HT sequence detection system (Applied Biosystems) was used to perform Taqman real-time quantitative PCR. Use Taqman Universal PCR Master Mix (Applied Biosystems) to run real-time PCR, and prepare each reaction according to the following table:
[0081] Table 1
[0082]
[0083] For each response, in Add 40 μL of the above master mix to 10 μL of DNA eluate on the Optical 96-well reaction plate (Cat#N8010560, Applied Biosystems), and subject it to PCR analysis according to the following steps:
[0084] 1.50℃ for 2 minutes-1 cycle
[0085] 2.95℃ for 10 minutes-1 cycle
[0086] 3.95°C for 15 seconds; 60°C for 1 minute to 50 cycles
[0087] Use JC virus (Ca...
Embodiment 3
[0097] Example 3: Comparison
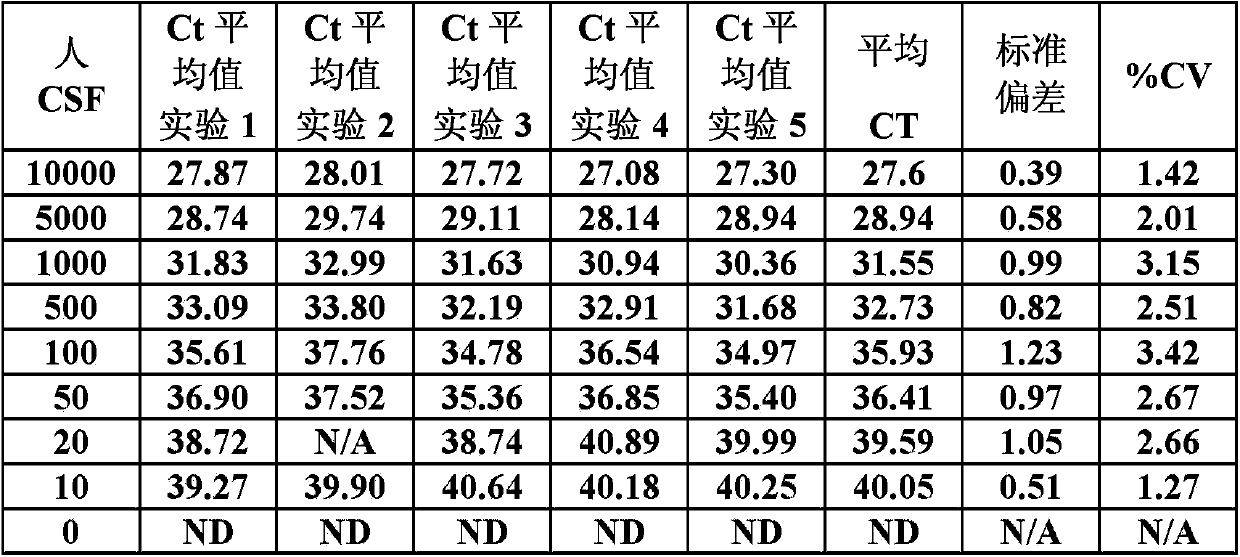
[0098] The results of the method described in Example 1 were compared with the method described in the "standard" protocol provided by QIAamp MinElute Virus Spin Kit (Cat#57704, Qiagen). See, for example, pages 59-60 of the DNA Mini Kit manual and pages 19-21 of the QIAamp MinElute Virus Spin Kit manual. Different amounts of JC virus DNA copies were added to the CSF sample, and the "standard" protocol and the protocol described in Example 1 were used to isolate the DNA. The RT-PCR protocol described in Example 2 was used to determine the copy number of JC virus DNA in a sample containing the isolated DNA.
[0099] The "standard" extraction method produces an assay sensitivity of 500 copies / mL. The method described in Example 1 produces a detection of 10 copies / mL. (See table below)
[0100] Table 4: Comparison between the method of Example 1 and the standard scheme
[0101]
[0102] Equivalent
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com