Kit and method for simultaneously detecting herpes simplex virus, Carbosarcoma associated herpes virus, JC virus and EB virus
A herpes simplex virus, herpes virus technology, applied in the field of real-time fluorescent PCR detection kits, can solve the problem of inability to detect herpes simplex virus Kaposi's sarcoma-related herpes virus, JC virus and EB virus at the same time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] [Example 1] A kit for detecting herpes simplex virus, Kaposi's sarcoma-associated herpes virus, JC virus and Epstein-Barr virus in the same reaction is provided.
[0069] The kit includes PCR reaction solution, nucleic acid composition for simultaneous detection of herpes simplex virus, Kaposi's sarcoma-associated herpes virus, JC virus and Epstein-Barr virus, hot start Taq enzyme, positive control and negative control. Wherein, the PCR reaction solution includes 250mM Tris-base, 0.25% TritonX-100, 25mmol / L MgCl 2 .
[0070] Wherein the nucleic acid composition comprises:
[0071] A pair of herpes simplex virus amplification primers whose sequences are shown in SEQ ID No.1 and SEQ ID No.2;
[0072] Kaposi's sarcoma-associated herpesvirus amplification primer pair whose sequences are shown in SEQ ID No.4 and SEQ ID No.5;
[0073] The JC virus amplification primer pair whose sequences are shown in SEQ ID No.7 and SEQ ID No.8;
[0074] The Epstein-Barr virus amplificat...
Embodiment 2
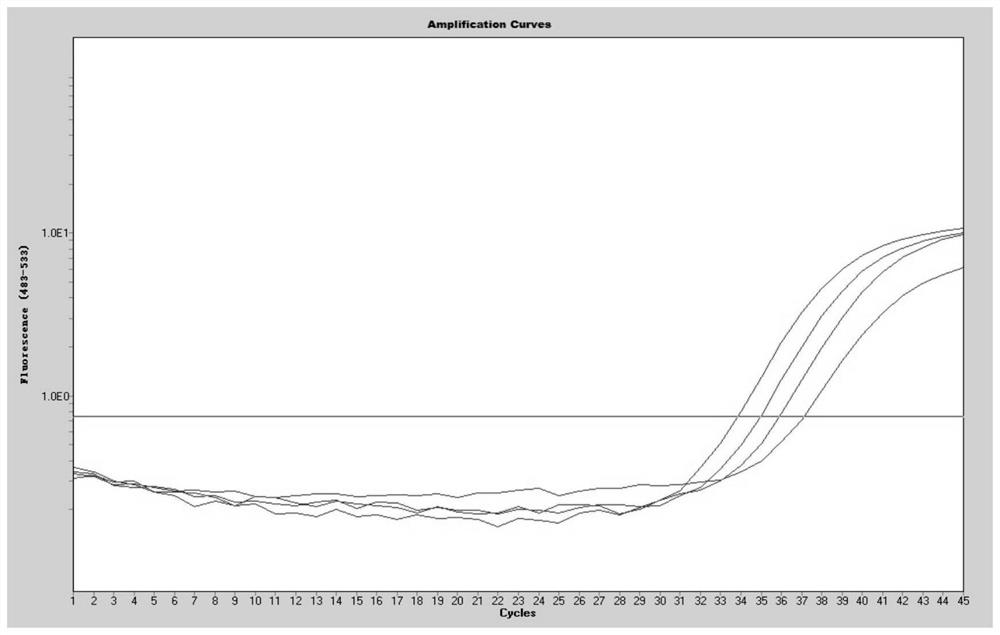
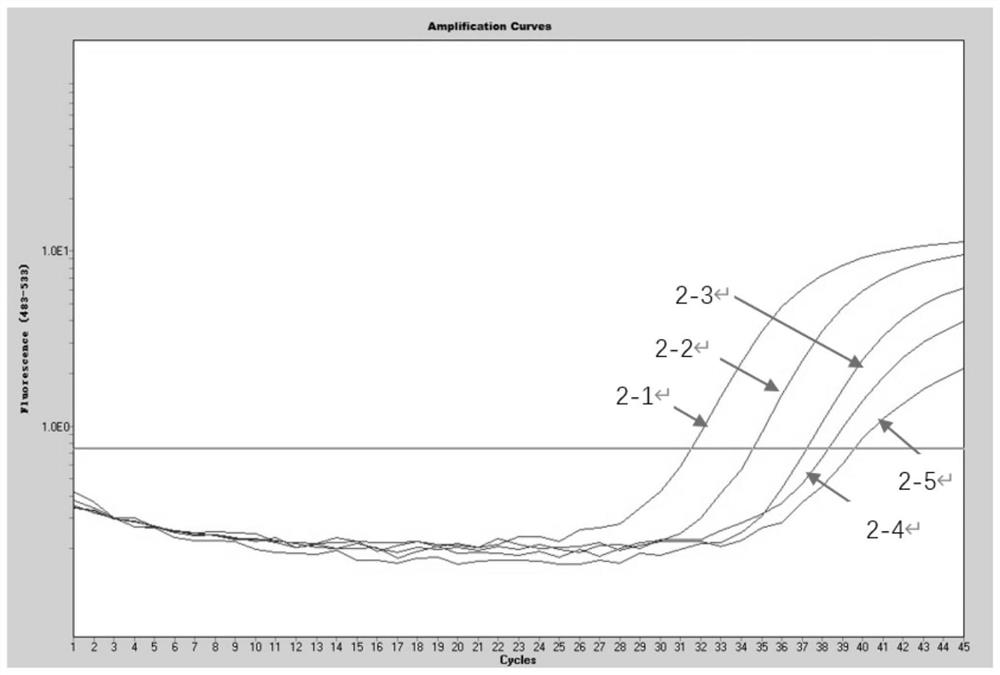
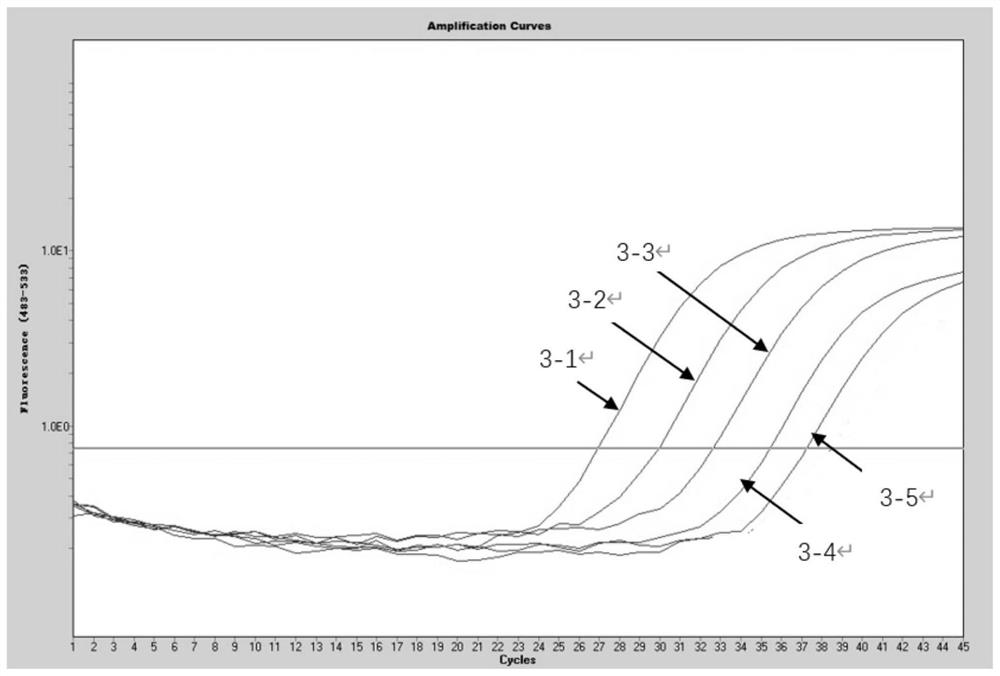
[0082] [Example 2] Determination of the sensitivity of the kit in Example 1 to detect four viruses in the same reaction
[0083] (1) Herpes simplex virus positive control substance, Kaposi's sarcoma-associated herpesvirus positive control substance, JC virus positive control substance, and EB virus positive control substance in the kit of Example 1 are mixed and made into a mixed solution to obtain positive to be treated Test product (the concentration of each virus positive control substance is 10 6 copies / mL).
[0084] (2) Perform a 10-fold serial dilution of the positive sample to be tested (i.e. 10copies / mL~10 5 copies / mL), using the kit of Example 1 to perform multiple fluorescent quantitative PCR detection on the positive test items under each gradient, the system of multiple fluorescent quantitative PCR reactions is shown in Table 1. The reaction conditions of the multiplex fluorescent quantitative PCR reaction were: pre-denaturation at 95°C for 3 min; denaturation at...
Embodiment 3
[0090] [Example 3] The specificity comparison between the kit of Example 1 and known commercialized kits
[0091] (1) Herpes simplex virus positive control substance, Kaposi's sarcoma-associated herpesvirus positive control substance, JC virus positive control substance, EB virus positive control substance in the test kit of embodiment 1 (the concentration of each virus positive control substance is equal to for 10 6 copies / mL) were mixed and made into a mixed solution to obtain a positive test article; physiological saline not containing the above four virus positive control substances was a negative test article.
[0092] (2) The experiment is divided into an experimental group, a control group 1 and a control group 2, and the kit of the experimental group is the kit of Example 1. The test kit of matched group 1 is roughly the same as the kit of embodiment 1, and the kit of matched group 2 is roughly the same as the kit of embodiment 1 (the kit components of matched group 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com