Recombinant human antibodies for therapy and prevention of polyomavirus-related diseases
a technology of polyomavirus and human antibodies, applied in the direction of immunomodulatory therapies, drug compositions, peptides, etc., can solve the problems of immunomodulatory therapies, lymphocytic leukemia (cll), crohn's disease, and an increased risk of pml inciden
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
of Blood Donors
[0350]Clinically interesting donors were recruited, peripheral blood was drawn under appropriate informed consent and PBMCs were prepared and stored in liquid nitrogen.
[0351]The blood donors can be divided in 3 categories including healthy elderly patients with unknown HLA typing and anti-JCV titers, HLA-DRB1*04:01+ healthy donors who presented a robust JCV-specific antibody production, and patients who received monoclonal antibody therapy to treat Multiple Sclerosis and who developed symptoms of and successfully recovered from PML and PML-IRIS. Selected donors from the latter category mounted an efficient immune response with high anti-JCV antibody titers.
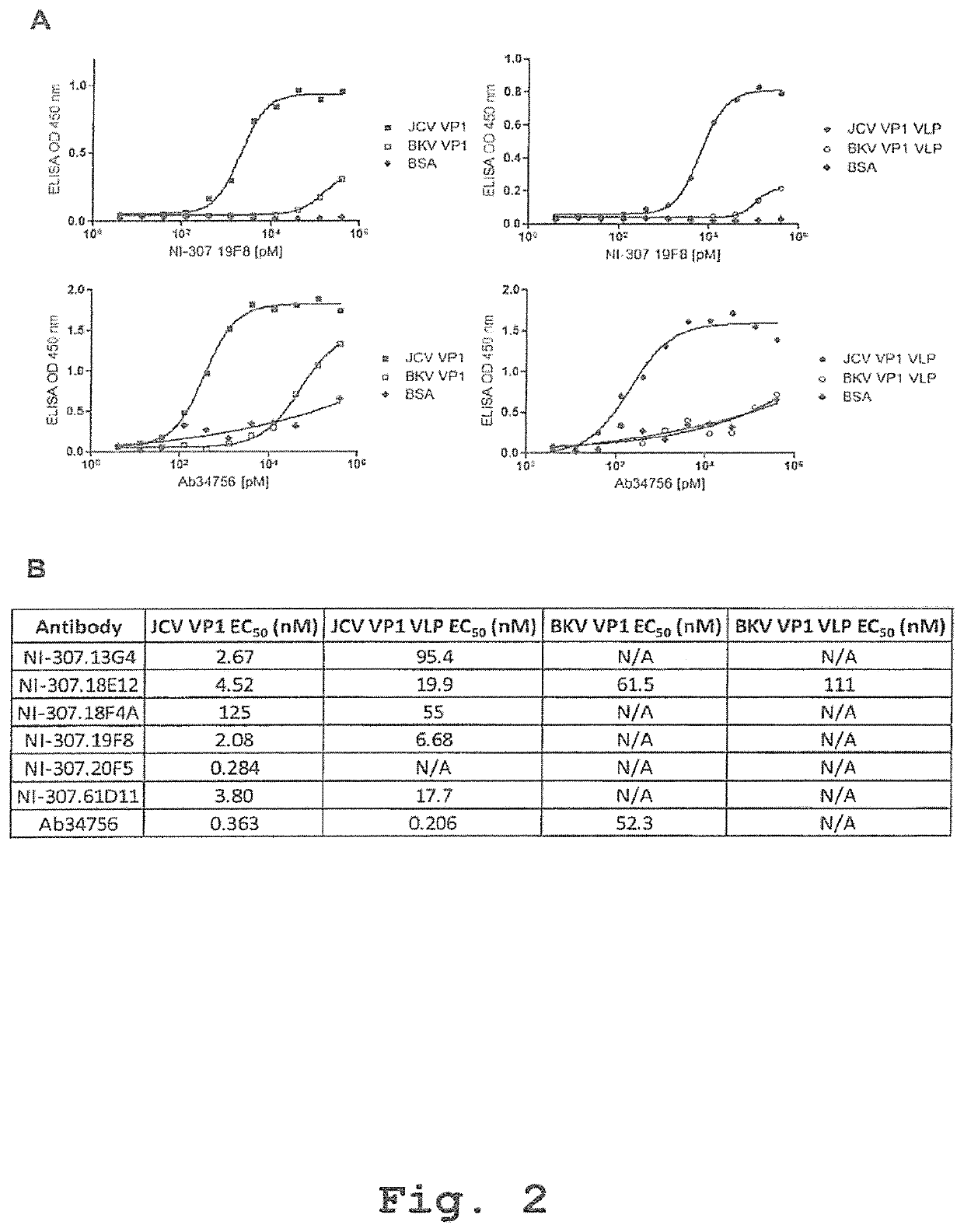
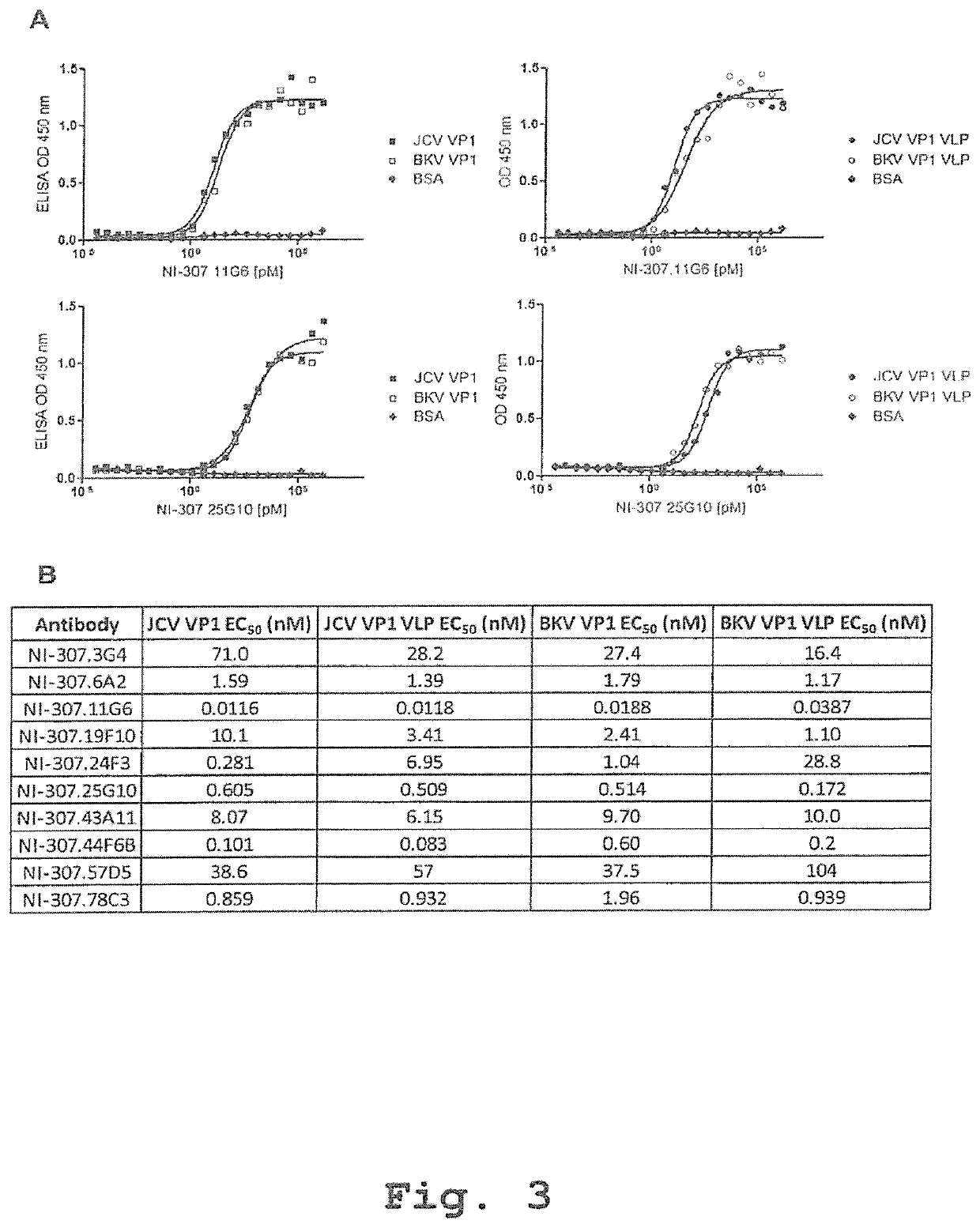
[0352]Human-derived antibodies targeting VP1 were identified by high-throughput analysis of complements of the human memory B-cell repertoire derived from the clinically selected donors. For VP1 antibody screening, 96-well microplates (Costar, Corning, USA) were coated overnight at 4° C. with VP1 solutions or BSA (S...
example 2
olding of VP1 Proteins into Virus-Like Particles
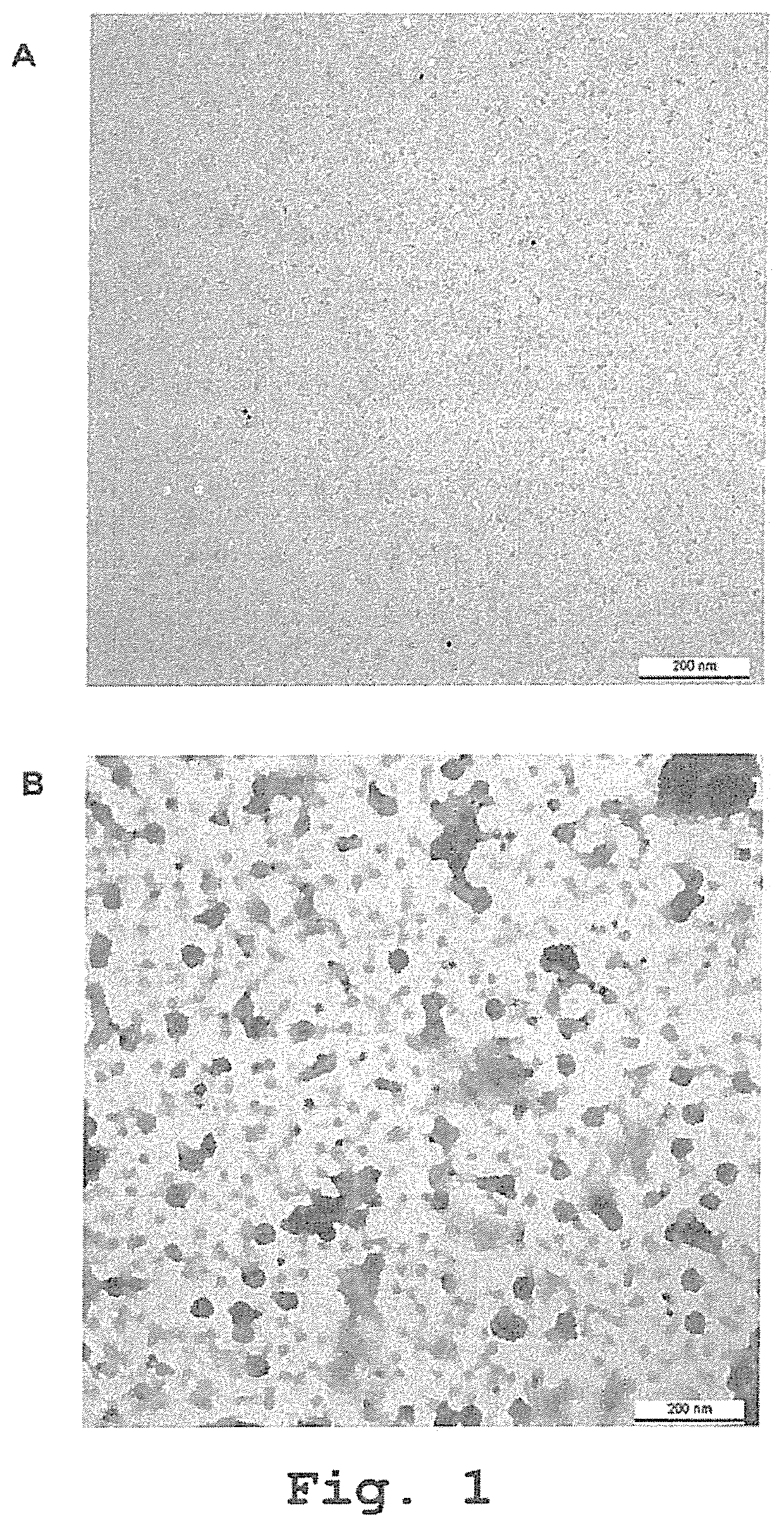
[0355]To screen and test the binding of the antibody hits to VP1 epitopes exposed on the surface of the viruses, the refolding of VP1 monomers into Virus-Like Particles (VLPs) had to be to set up. VP1 proteins (Abcam) were either diluted in carbonate ELISA coating buffer or incubated for 48 h at 24° C. in reassociation buffer (TBS containing 1 mM CaCl2). The preparations were then stained with an anti-VP1 antibody and studied by transmission electron microscopy. As shown in FIG. 1A, the VP1 proteins diluted in carbonate coating buffer do not form any structure. On the contrary, VP1 proteins incubated in reassociation buffer are reassembling into VLPs mimicking the structure of the viruses (see FIG. 1B). This procedure was further used to refold the particles to be coated on ELISA plates (referred as VP1 VLPs).
example 3
Cloning of VP1 Antibodies
[0356]Living B cells of selected memory B cell cultures were harvested and mRNA was prepared. Immunoglobulin heavy and light chain sequences were then obtained using a nested PCR approach.
[0357]A combination of primers representing all sequence families of the human immunoglobulin germline repertoire was used for the amplifications of leader peptides, V-segments and J-segments. The first round of amplification was performed using leader peptide-specific primers in 5′-end and constant region-specific primers in 3′-end (Smith et al., Nat Protoc. 4 (2009), 372-384). For heavy chains and kappa light chains, the second round of amplification was performed using V-segment-specific primers at the 5′-end and J-segment-specific primers at the 3′end. For lambda light chains, the second round amplification was performed using V-segment-specific primers at the 5′-end and a C-region-specific primer at the 3′end (Marks et al., Mol. Biol. 222 (1991), 581-597; de Haard et a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com