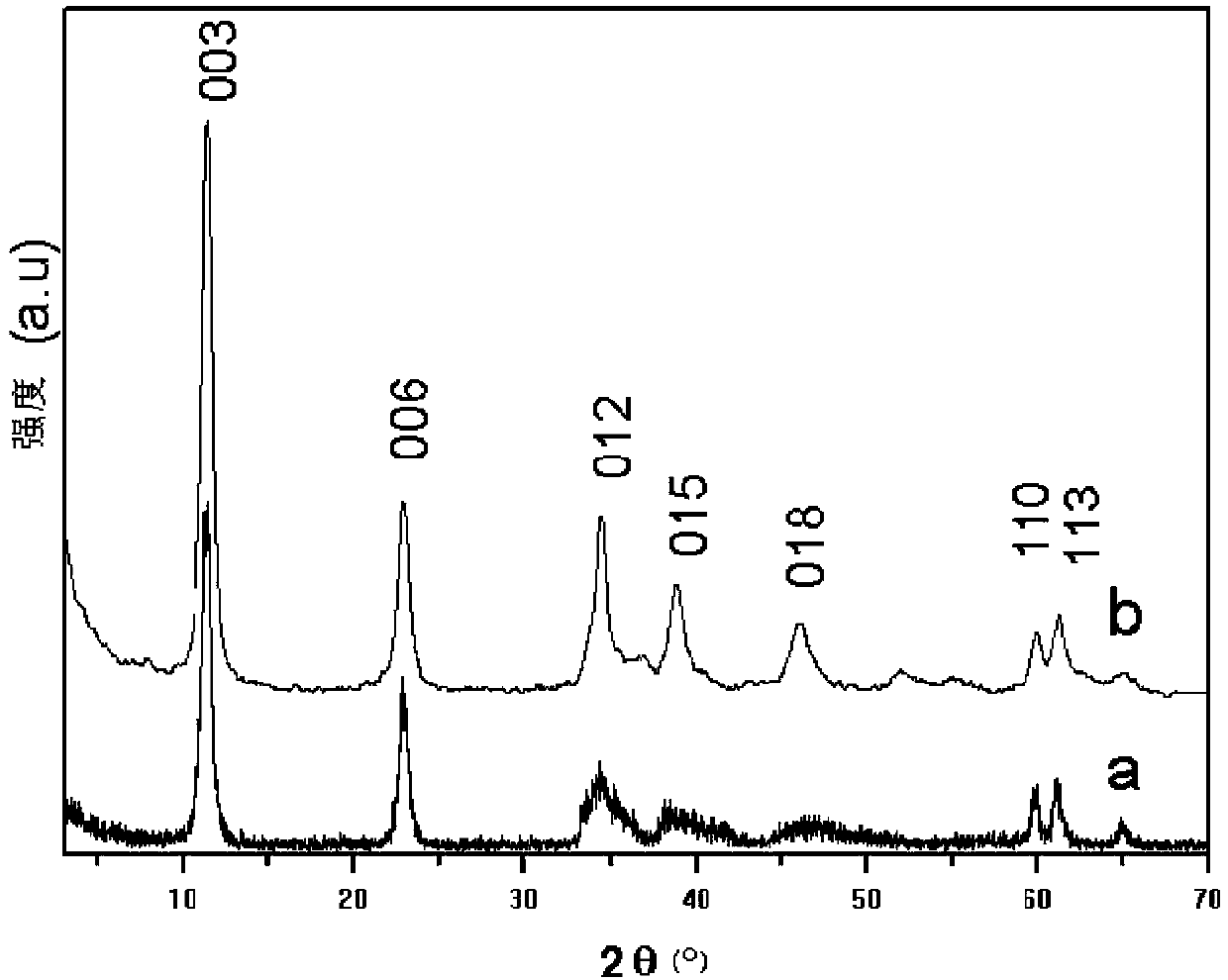
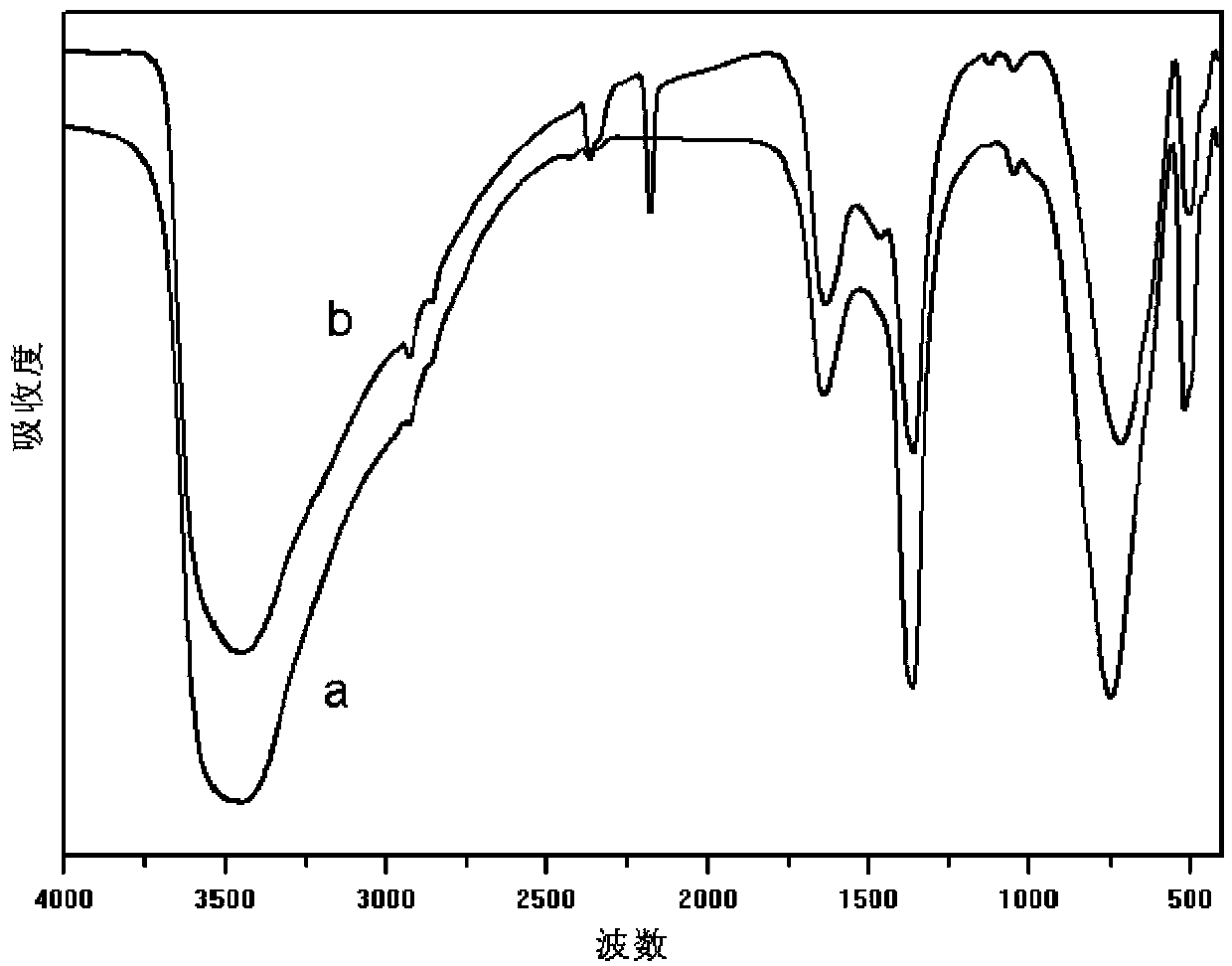
Preparation method of high-crystallinity Fe-based hydrotalcite-like compound
A hydrotalcite and iron-based technology, which is applied in chemical instruments and methods, iron compounds, inorganic chemistry, etc., can solve problems that cannot effectively meet metal ion reaction conditions, limitations, and complex reaction processes, and achieve simple operation and mild process conditions , The effect of simplifying the preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The preparation of embodiment 1 nickel-iron-based hydrotalcite
[0026] Step A: 5.4527g (0.0189mol) of solid Ni(NO 3 ) 2 ·6H 2 O and 2.5250g (0.0063mol) of solid Fe(NO 3 ) 3 9H 2 O is dissolved in 50ml deionized water, and stirred to make it dissolve to obtain a mixed solution, the total metal ion concentration (Ni 2+ +Fe 3+ ) is 0.5mol / L, and the molar ratio of nickel and iron ions is 3:1.
[0027] Step B: Add 17.0270 g (0.2835 mol) of urea to the mixed solution obtained in Step A, the molar concentration ratio of urea to nitrate ions in the mixed solution is 5:1, stir for 3-5 minutes until the urea is completely dissolved in the mixed solution .
[0028] Step C: Transfer the mixed solution added with urea to a polytetrafluoroethylene liner of a 100ml hydrothermal synthesis reactor, tighten the reactor and place it in an oven. The hydrothermal reaction was carried out at 90° C., and the reaction time was 72 hours. After the reaction is completed, the reaction...
Embodiment 2
[0032] The preparation of embodiment 2 nickel-iron-based hydrotalcites
[0033] Step A: 5.4527g (0.0189mol) of solid Ni(NO 3 ) 2 ·6H 2 O and 2.5250g (0.0063mol) of solid Fe(NO 3 ) 3 9H 2 O is dissolved in 25ml deionized water, stirred to make it dissolve to obtain a mixed solution, the total metal ion concentration (Ni 2+ +Fe 3+ ) is 1.0mol / L, and the molar ratio of nickel and iron ions is 3:1.
[0034] Step B: Add urea-27.2432g (0.4536mol) to the mixed solution obtained in step A, the molar concentration ratio of urea to nitrate ions in the mixed solution is 8:1, stir for 3-5 minutes until the urea is completely dissolved in the mixed solution middle.
[0035] Step C: Transfer the mixed solution added with urea to a polytetrafluoroethylene liner of a 100ml hydrothermal synthesis reactor, tighten the reactor and place it in an oven. The hydrothermal reaction was carried out at 120° C., and the reaction time was 48 hours. After the reaction is completed, the reaction ...
Embodiment 3
[0037] The preparation of embodiment 3 nickel-iron-based hydrotalcites
[0038] Step A: 3.6351g (0.0126mol) of solid Ni(NO 3 ) 2 ·6H 2 O and 2.5250g (0.0063mol) of solid Fe(NO 3 ) 3 9H 2 O is dissolved in 100ml deionized water, stirred to make it dissolve to obtain a mixed solution, the total metal ion concentration (Ni 2+ +Fe 3+ ) is 0.25mol / L, and the molar ratio of nickel and iron ions is 2:1.
[0039] Step B: Add 10.2162 g (0.1701 mol) of urea to the mixed solution obtained in Step A, the molar concentration ratio of urea to nitrate ions in the mixed solution is 3:1, stir for 3-5 minutes until the urea is completely dissolved in the mixed solution .
[0040] Step C: Transfer the mixed solution added with urea to a polytetrafluoroethylene liner of a 200ml hydrothermal synthesis reactor, tighten the reactor and place it in an oven. The hydrothermal reaction was carried out at 80° C., and the reaction time was 60 hours. After the reaction is completed, the reaction ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com