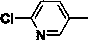Dithiolane-containing cis-anabasine compound and preparation method and application thereof
A compound and composition technology, applied in the field of new neonicotinoid insecticides, can solve the problems of severe resistance, narrow insecticidal spectrum, and limited selectivity of insect pest control drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Embodiment 1. Synthesis of compound 1
[0051]
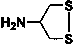
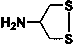
[0052] 2-Chloro-5-(2-nitromethylene-imidazolidin-1-ylmethyl)-pyridine (5 mmol), 4-amino-1,2-dithiolane (5.5 mmol), 37 % formaldehyde solution (11 mmol) was added to a 50 mL round bottom flask, and stirred overnight at room temperature. Filter with suction and spin dry the solvent to obtain the crude product. Further separation by column chromatography (dichloromethane: methanol = 10:1) gave a light yellow powdery solid with a yield of about 63%. 1 H NMR (400 Mz, CDCl 3 ): δ 8.26 (d, J = 2.2 Hz, 1H), 7.54 (dd, J = 8.0 Hz, J = 2.2 Hz , 1H), 7.36 (d, J = 8.1 Hz, 1H), 4.90 (d, J = 15.0 Hz, 1H), 4.53 (d, J = 15.0 Hz, 1H), 3.72-3.92 (m, 4H), 3.68 (s, 4H), 2.92 (m, 1H), 2.56-2.75 (m, 4H); HRMS(ES+) calculated value C 15 h 19 N 5 o 2 S 2 35 Cl(M+H)+, 400.0669; Found, 400.0661.
[0053]
Embodiment 2
[0054] Example 2. Synthesis of compound 2
[0055]
[0056] 1-((2-chlorothiazol-5-yl)methyl)-2-(nitromethylene)-1-imidazolidine (5 mmol), 4-amino-1,2-dithiolane (5.5 mmol), 37% formaldehyde aqueous solution (11 mmol) were added into a 50 mL round bottom flask, and stirred overnight at room temperature. Filter with suction and spin dry the solvent to obtain the crude product. Further separation by column chromatography (dichloromethane: methanol = 10:1) gave a light yellow powdery solid with a yield of about 52%. 1 H NMR (400 Mz, CDCl 3 ): δ 6.72 (s, 1H), 4.83 (d, J = 15.0 Hz, 1H), 4.38 (d, J = 15.0 Hz, 1H), 3.65-3.83 (m, 4H), 3.61 (s, 4H), 2.90 (m, 1H), 2.48-2.72 (m, 4H); HRMS(ES+) calculated value C 13 h 17 N 5 o 2 S 3 35 Cl(M+H)+, 406.0233; Found, 406.0237.
[0057]
Embodiment 3
[0058] Example 3. Synthesis of compound 3
[0059]
[0060] Nitenpyram (5 mmol), 4-amino-1,2-dithiolane (5.5 mmol), and 37% aqueous formaldehyde (11 mmol) were added into a 50 mL round bottom flask, and stirred overnight at room temperature. Filter with suction and spin dry the solvent to obtain the crude product. Further separation by column chromatography (dichloromethane: methanol = 10:1) gave a light yellow powdery solid with a yield of about 32%. 1 H NMR (400 Mz, CDCl 3 ): δ 8.32 (d, J = 2.2 Hz, 1H), 7.72 (dd, J = 8.0 Hz, J = 2.2 Hz , 1H), 7.34 (d, J = 8.1 Hz, 1H), 4.50 (d, J = 15.0 Hz, 1H), 4.41 (d, J = 15.0 Hz, 1H), 3.68-3.89 (m, 4H), 3.70 (s, 4H), 2.88 (m, 1H), 2.46-2.73 (m, 4H); HRMS(ES+) calculated value C 16 h 23 N 5 o 2 S 2 35 Cl(M+H)+, 416.0982; Found, 416.0978.
[0061]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com